Potassium »
PDB 2adp-2c13 »
2ats »
Potassium in PDB 2ats: Dihydrodipicolinate Synthase Co-Crystallised with (S)-Lysine
Enzymatic activity of Dihydrodipicolinate Synthase Co-Crystallised with (S)-Lysine
All present enzymatic activity of Dihydrodipicolinate Synthase Co-Crystallised with (S)-Lysine:
4.2.1.52;
4.2.1.52;
Protein crystallography data
The structure of Dihydrodipicolinate Synthase Co-Crystallised with (S)-Lysine, PDB code: 2ats
was solved by
S.R.A.Devenish,
R.C.J.Dobson,
G.B.Jameson,
J.A.Gerrard,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 25.80 / 1.90 |
| Space group | P 31 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 121.680, 121.680, 109.799, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 16.8 / 20.2 |
Other elements in 2ats:
The structure of Dihydrodipicolinate Synthase Co-Crystallised with (S)-Lysine also contains other interesting chemical elements:
| Chlorine | (Cl) | 2 atoms |
Potassium Binding Sites:
The binding sites of Potassium atom in the Dihydrodipicolinate Synthase Co-Crystallised with (S)-Lysine
(pdb code 2ats). This binding sites where shown within
5.0 Angstroms radius around Potassium atom.
In total 2 binding sites of Potassium where determined in the Dihydrodipicolinate Synthase Co-Crystallised with (S)-Lysine, PDB code: 2ats:
Jump to Potassium binding site number: 1; 2;
In total 2 binding sites of Potassium where determined in the Dihydrodipicolinate Synthase Co-Crystallised with (S)-Lysine, PDB code: 2ats:
Jump to Potassium binding site number: 1; 2;
Potassium binding site 1 out of 2 in 2ats
Go back to
Potassium binding site 1 out
of 2 in the Dihydrodipicolinate Synthase Co-Crystallised with (S)-Lysine
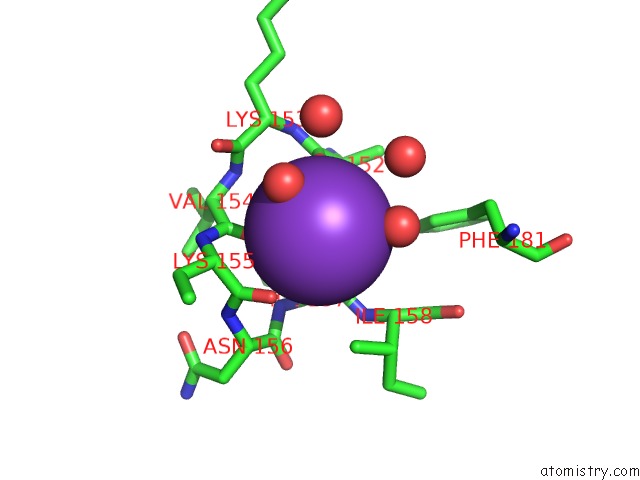
Mono view
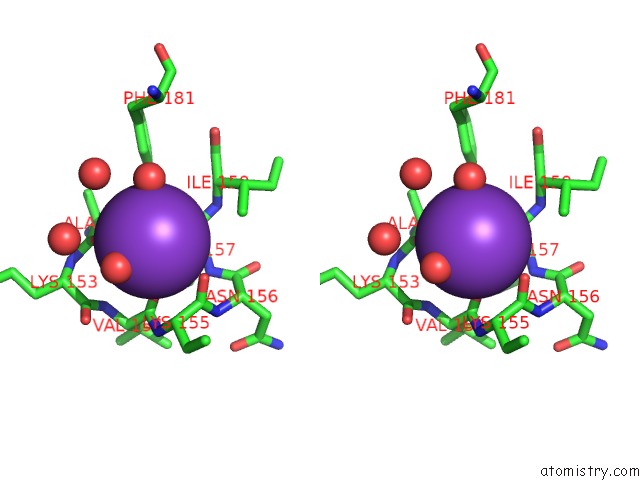
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 1 of Dihydrodipicolinate Synthase Co-Crystallised with (S)-Lysine within 5.0Å range:
|
Potassium binding site 2 out of 2 in 2ats
Go back to
Potassium binding site 2 out
of 2 in the Dihydrodipicolinate Synthase Co-Crystallised with (S)-Lysine
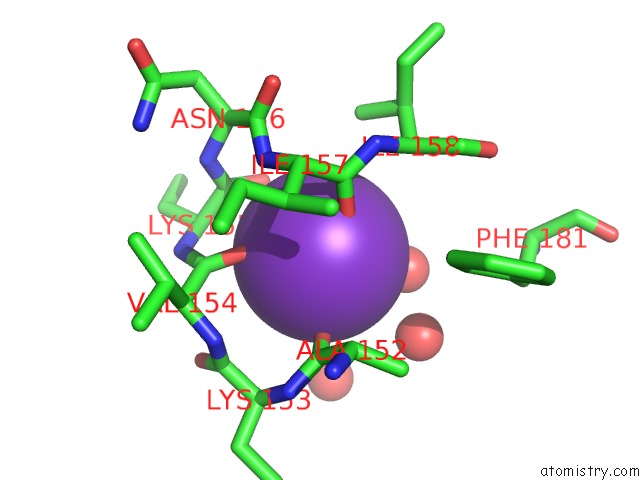
Mono view
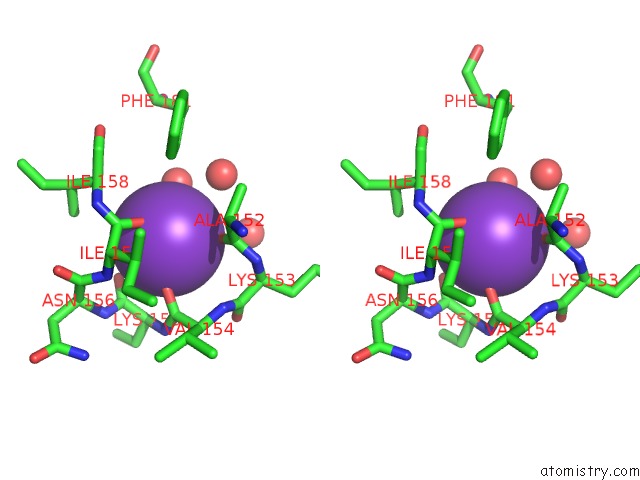
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 2 of Dihydrodipicolinate Synthase Co-Crystallised with (S)-Lysine within 5.0Å range:
|
Reference:
S.R.A.Devenish,
R.C.J.Dobson,
G.B.Jameson,
J.A.Gerrard.
The Co-Crystallisation of (S)-Lysine-Bound Dihydrodipicolinate Synthase From E. Coli Indicates That Domain Movements Are Not Responsible For (S)-Lysine Inhibition To Be Published.
Page generated: Sat Aug 9 03:07:54 2025
Last articles
K in 7QIYK in 7Q3X
K in 7QDN
K in 7QF6
K in 7Q0G
K in 7Q1C
K in 7Q1B
K in 7PXH
K in 7PZJ
K in 7PXG