Potassium »
PDB 9gku-9mek »
9gn1 »
Potassium in PDB 9gn1: Crystal Structure of Inactive Deacetylase (Hdah) H144A From Klebsiella Pneumoniae Subsp. Ozaenae
Protein crystallography data
The structure of Crystal Structure of Inactive Deacetylase (Hdah) H144A From Klebsiella Pneumoniae Subsp. Ozaenae, PDB code: 9gn1
was solved by
Q.Qin,
L.G.Graf,
S.Schulze,
G.J.Palm,
M.Lammers,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 46.25 / 2.35 |
| Space group | I 2 3 |
| Cell size a, b, c (Å), α, β, γ (°) | 146.117, 146.117, 146.117, 90, 90, 90 |
| R / Rfree (%) | 15.3 / 20.4 |
Other elements in 9gn1:
The structure of Crystal Structure of Inactive Deacetylase (Hdah) H144A From Klebsiella Pneumoniae Subsp. Ozaenae also contains other interesting chemical elements:
| Zinc | (Zn) | 2 atoms |
Potassium Binding Sites:
The binding sites of Potassium atom in the Crystal Structure of Inactive Deacetylase (Hdah) H144A From Klebsiella Pneumoniae Subsp. Ozaenae
(pdb code 9gn1). This binding sites where shown within
5.0 Angstroms radius around Potassium atom.
In total 2 binding sites of Potassium where determined in the Crystal Structure of Inactive Deacetylase (Hdah) H144A From Klebsiella Pneumoniae Subsp. Ozaenae, PDB code: 9gn1:
Jump to Potassium binding site number: 1; 2;
In total 2 binding sites of Potassium where determined in the Crystal Structure of Inactive Deacetylase (Hdah) H144A From Klebsiella Pneumoniae Subsp. Ozaenae, PDB code: 9gn1:
Jump to Potassium binding site number: 1; 2;
Potassium binding site 1 out of 2 in 9gn1
Go back to
Potassium binding site 1 out
of 2 in the Crystal Structure of Inactive Deacetylase (Hdah) H144A From Klebsiella Pneumoniae Subsp. Ozaenae
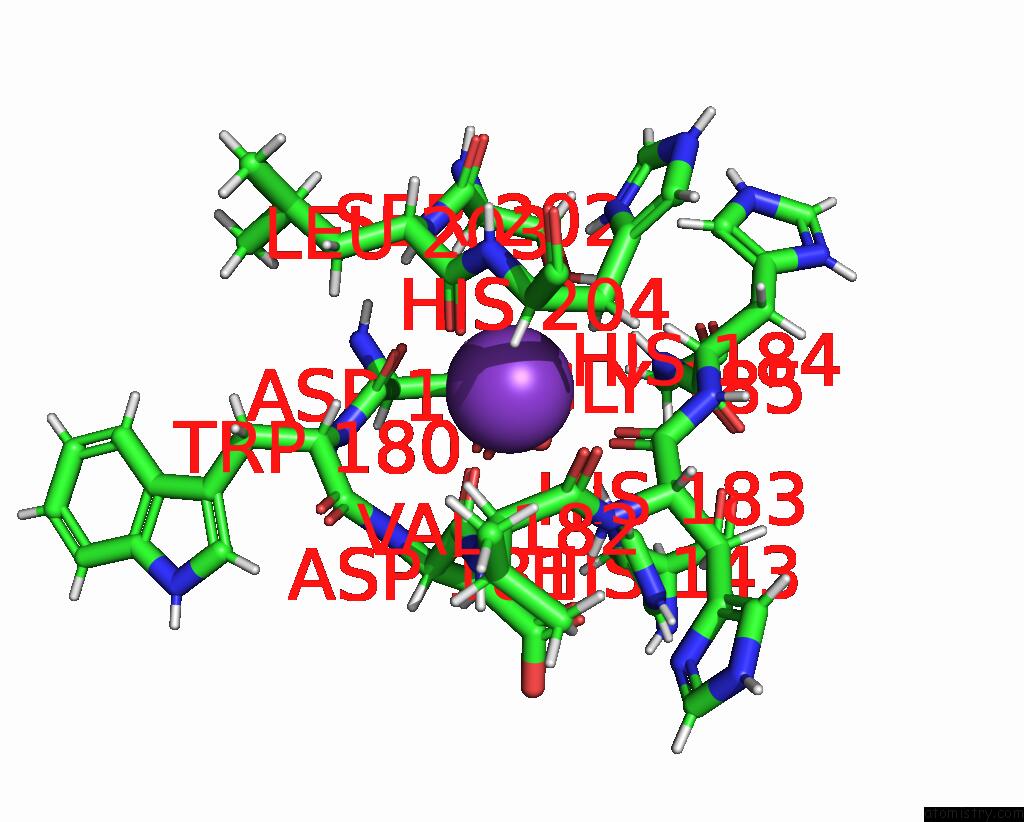
Mono view
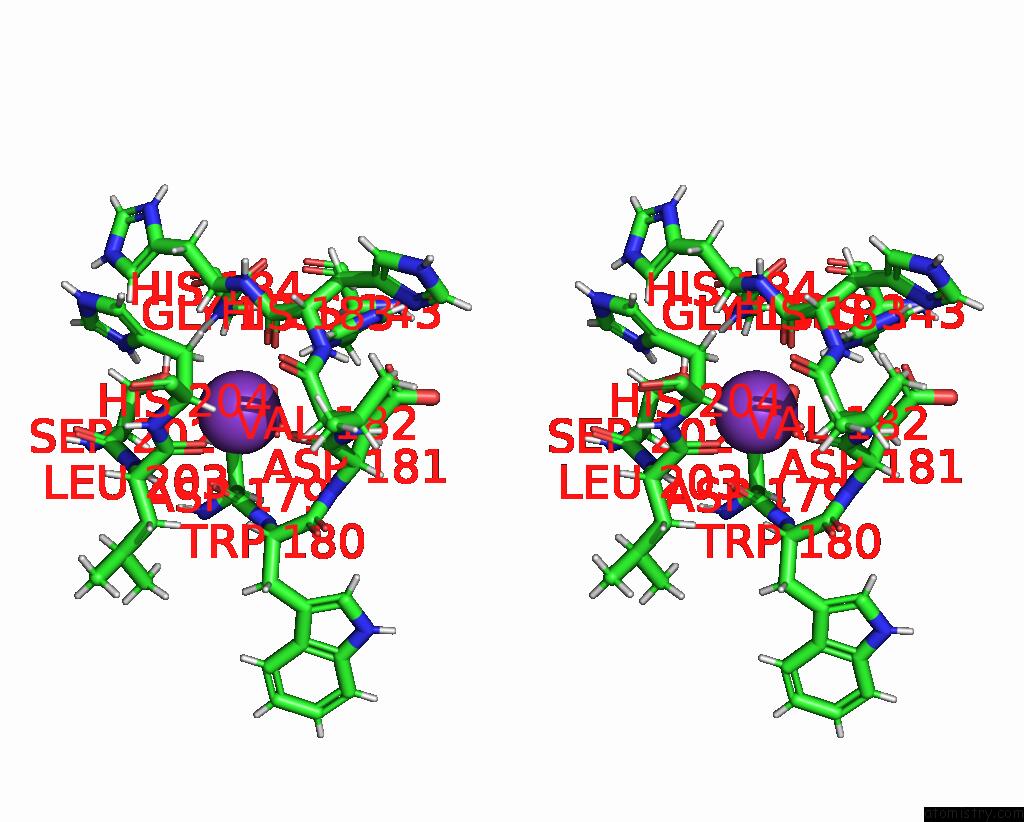
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 1 of Crystal Structure of Inactive Deacetylase (Hdah) H144A From Klebsiella Pneumoniae Subsp. Ozaenae within 5.0Å range:
|
Potassium binding site 2 out of 2 in 9gn1
Go back to
Potassium binding site 2 out
of 2 in the Crystal Structure of Inactive Deacetylase (Hdah) H144A From Klebsiella Pneumoniae Subsp. Ozaenae
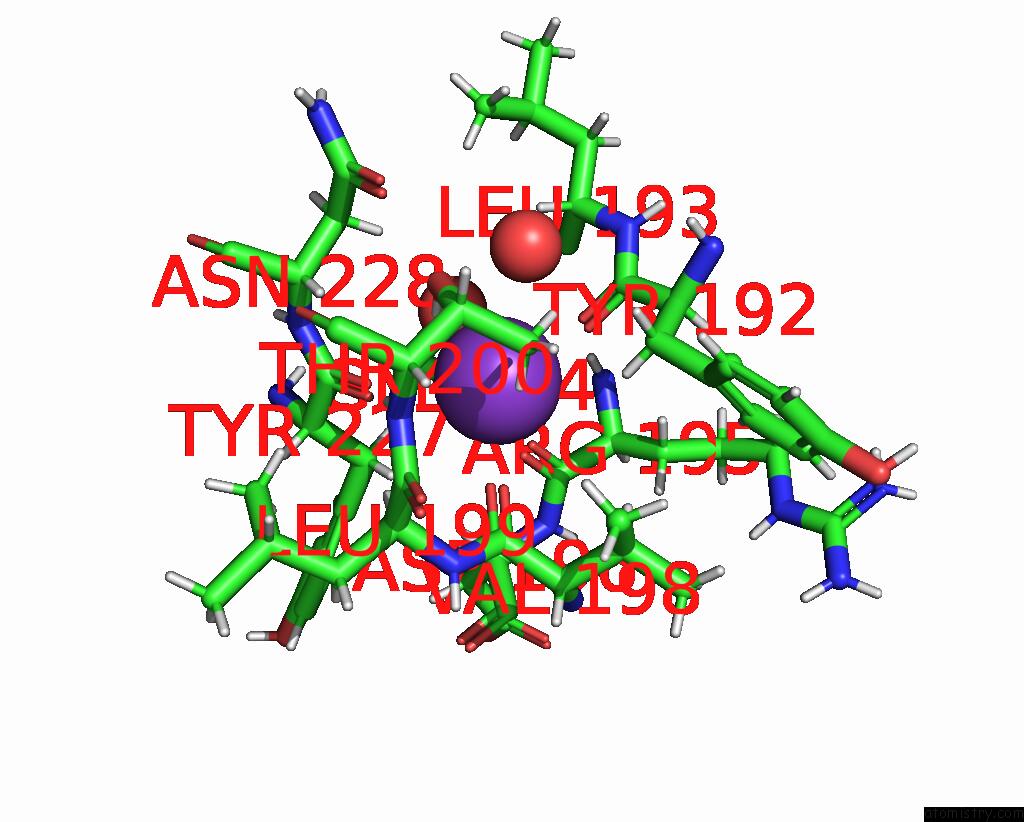
Mono view
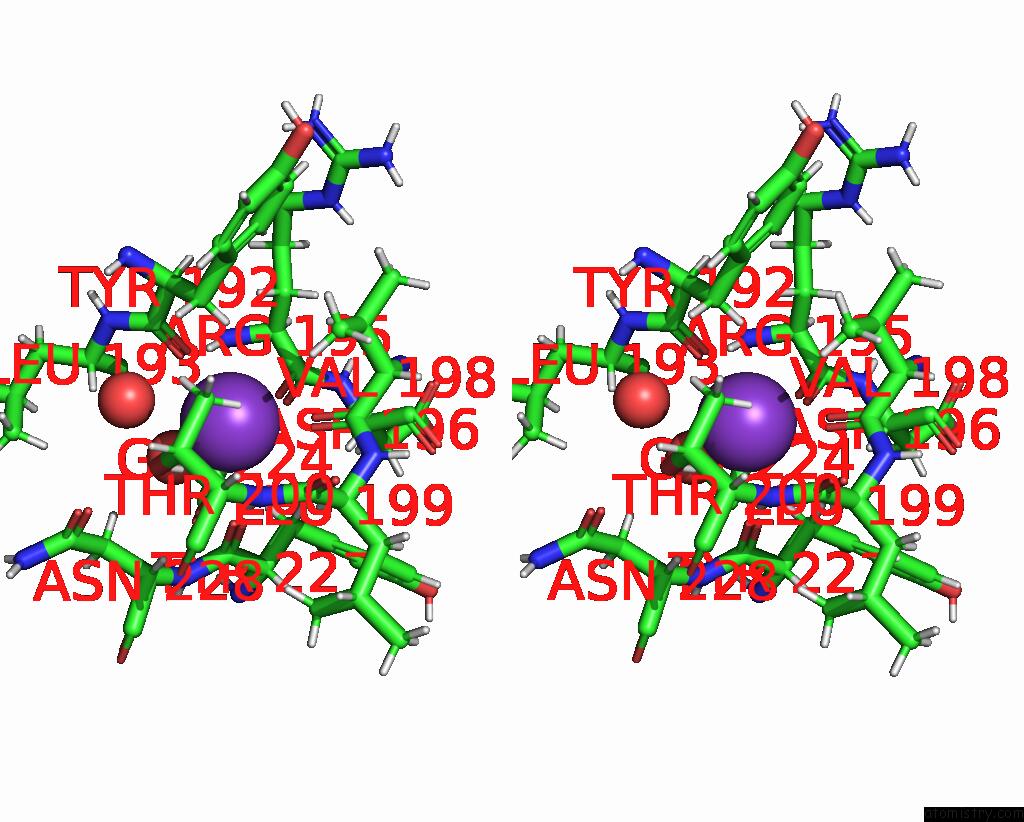
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 2 of Crystal Structure of Inactive Deacetylase (Hdah) H144A From Klebsiella Pneumoniae Subsp. Ozaenae within 5.0Å range:
|
Reference:
L.G.Graf,
C.Moreno-Yruela,
C.Qin,
S.Schulze,
G.J.Palm,
O.Schmoeker,
N.Wang,
D.Hocking,
L.Jebeli,
B.Girbardt,
L.Berndt,
D.M.Weis,
M.Janetzky,
D.Zuehlke,
S.Sievers,
R.A.Strugnell,
C.A.Olsen,
K.Hofmann,
M.Lammers.
Distribution and Diversity of Classical Deacylases in Bacteria Nature Communications 2024.
Page generated: Sat Aug 9 19:09:37 2025
Last articles
Mg in 9K3QMg in 9KOK
Mg in 9KBJ
Mg in 9KLN
Mg in 9KLQ
Mg in 9KK6
Mg in 9KI0
Mg in 9KHQ
Mg in 9KBI
Mg in 9KEV