Potassium »
PDB 4zun-5avw »
5a0y »
Potassium in PDB 5a0y: Methyl-Coenzyme M Reductase From Methanothermobacter Marburgensis at 1.1 A Resolution
Enzymatic activity of Methyl-Coenzyme M Reductase From Methanothermobacter Marburgensis at 1.1 A Resolution
All present enzymatic activity of Methyl-Coenzyme M Reductase From Methanothermobacter Marburgensis at 1.1 A Resolution:
2.8.4.1;
2.8.4.1;
Protein crystallography data
The structure of Methyl-Coenzyme M Reductase From Methanothermobacter Marburgensis at 1.1 A Resolution, PDB code: 5a0y
was solved by
T.Wagner,
U.Ermler,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 48.35 / 1.10 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 82.227, 118.300, 122.560, 90.00, 91.90, 90.00 |
| R / Rfree (%) | 11.1 / 12.9 |
Other elements in 5a0y:
The structure of Methyl-Coenzyme M Reductase From Methanothermobacter Marburgensis at 1.1 A Resolution also contains other interesting chemical elements:
| Nickel | (Ni) | 2 atoms |
| Magnesium | (Mg) | 16 atoms |
| Chlorine | (Cl) | 2 atoms |
| Sodium | (Na) | 2 atoms |
Potassium Binding Sites:
The binding sites of Potassium atom in the Methyl-Coenzyme M Reductase From Methanothermobacter Marburgensis at 1.1 A Resolution
(pdb code 5a0y). This binding sites where shown within
5.0 Angstroms radius around Potassium atom.
In total only one binding site of Potassium was determined in the Methyl-Coenzyme M Reductase From Methanothermobacter Marburgensis at 1.1 A Resolution, PDB code: 5a0y:
In total only one binding site of Potassium was determined in the Methyl-Coenzyme M Reductase From Methanothermobacter Marburgensis at 1.1 A Resolution, PDB code: 5a0y:
Potassium binding site 1 out of 1 in 5a0y
Go back to
Potassium binding site 1 out
of 1 in the Methyl-Coenzyme M Reductase From Methanothermobacter Marburgensis at 1.1 A Resolution
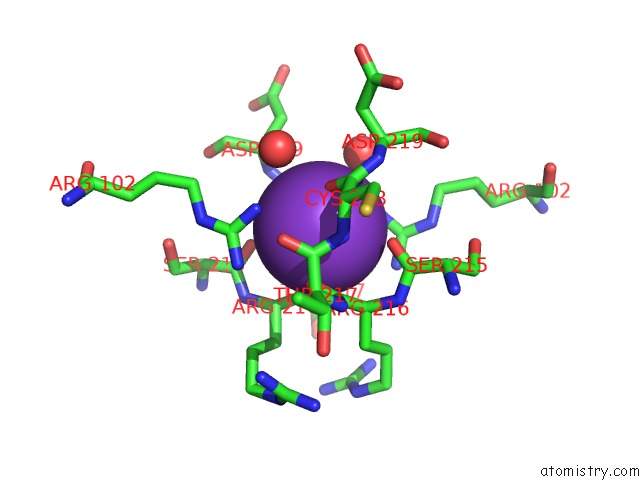
Mono view
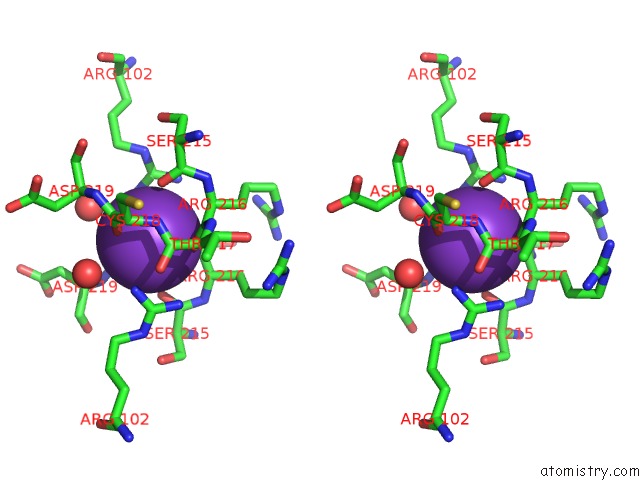
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 1 of Methyl-Coenzyme M Reductase From Methanothermobacter Marburgensis at 1.1 A Resolution within 5.0Å range:
|
Reference:
T.Wagner,
J.Kahnt,
U.Ermler,
S.Shima.
Didehydroaspartate Modification in Methyl-Coenzyme M Reductase Catalyzing Methane Formation. Angew.Chem.Int.Ed.Engl. V. 55 10630 2016.
ISSN: ISSN 1433-7851
PubMed: 27467699
DOI: 10.1002/ANIE.201603882
Page generated: Mon Aug 12 12:50:48 2024
ISSN: ISSN 1433-7851
PubMed: 27467699
DOI: 10.1002/ANIE.201603882
Last articles
F in 4WFAF in 4WF6
F in 4WF4
F in 4WEY
F in 4WEF
F in 4WET
F in 4WEV
F in 4WEG
F in 4W6Z
F in 4V0S