Potassium »
PDB 9byi-9djv »
9dic »
Potassium in PDB 9dic: Apo Aplysia SLO1 - R196Q
Potassium Binding Sites:
The binding sites of Potassium atom in the Apo Aplysia SLO1 - R196Q
(pdb code 9dic). This binding sites where shown within
5.0 Angstroms radius around Potassium atom.
In total 5 binding sites of Potassium where determined in the Apo Aplysia SLO1 - R196Q, PDB code: 9dic:
Jump to Potassium binding site number: 1; 2; 3; 4; 5;
In total 5 binding sites of Potassium where determined in the Apo Aplysia SLO1 - R196Q, PDB code: 9dic:
Jump to Potassium binding site number: 1; 2; 3; 4; 5;
Potassium binding site 1 out of 5 in 9dic
Go back to
Potassium binding site 1 out
of 5 in the Apo Aplysia SLO1 - R196Q
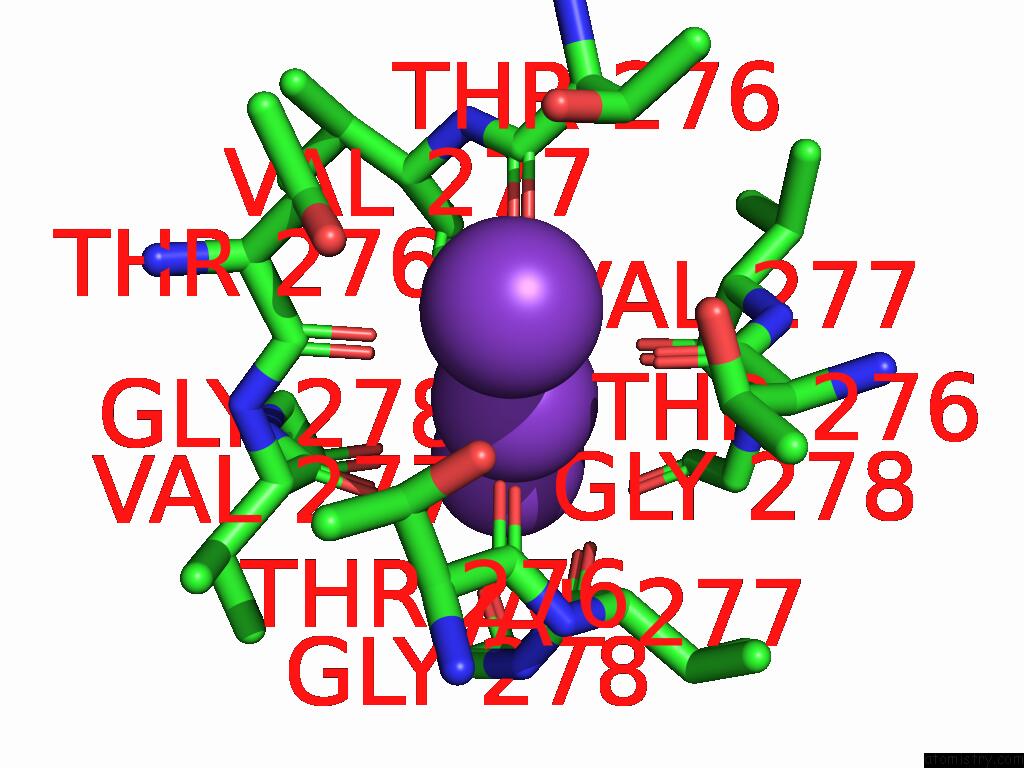
Mono view
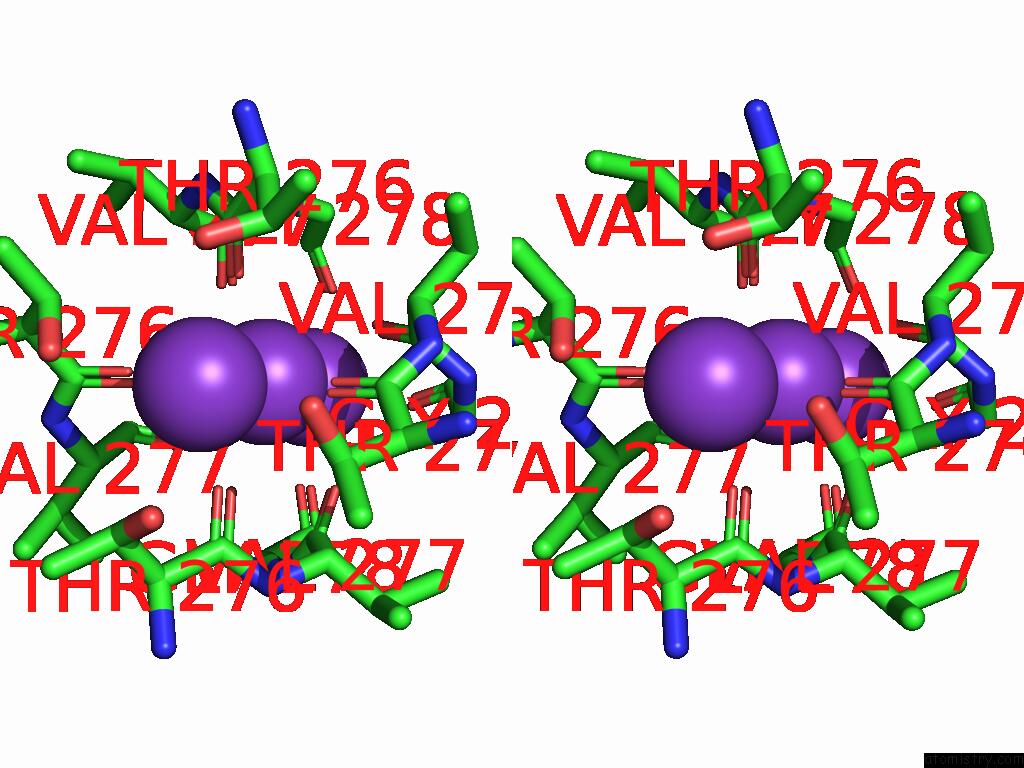
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 1 of Apo Aplysia SLO1 - R196Q within 5.0Å range:
|
Potassium binding site 2 out of 5 in 9dic
Go back to
Potassium binding site 2 out
of 5 in the Apo Aplysia SLO1 - R196Q
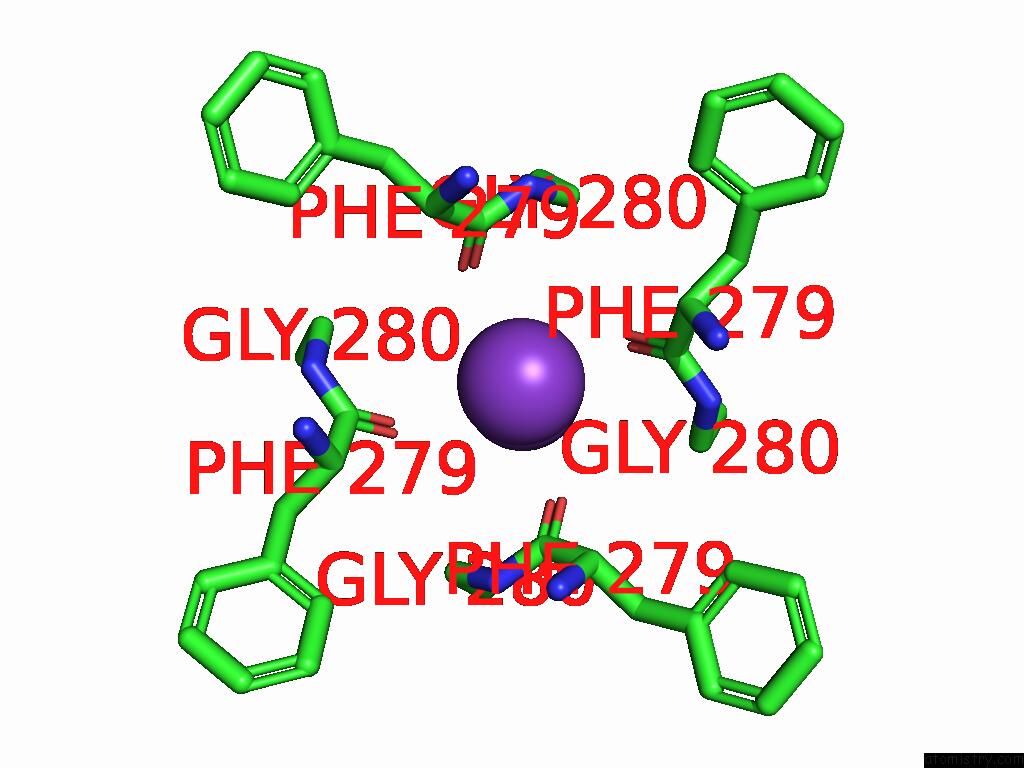
Mono view
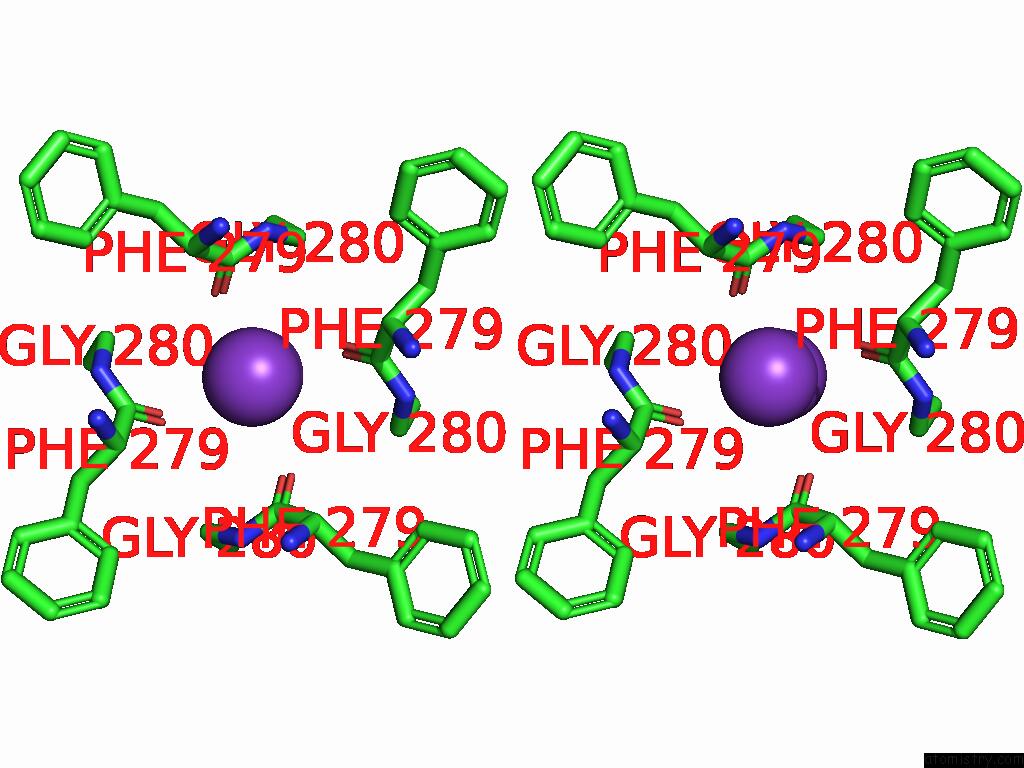
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 2 of Apo Aplysia SLO1 - R196Q within 5.0Å range:
|
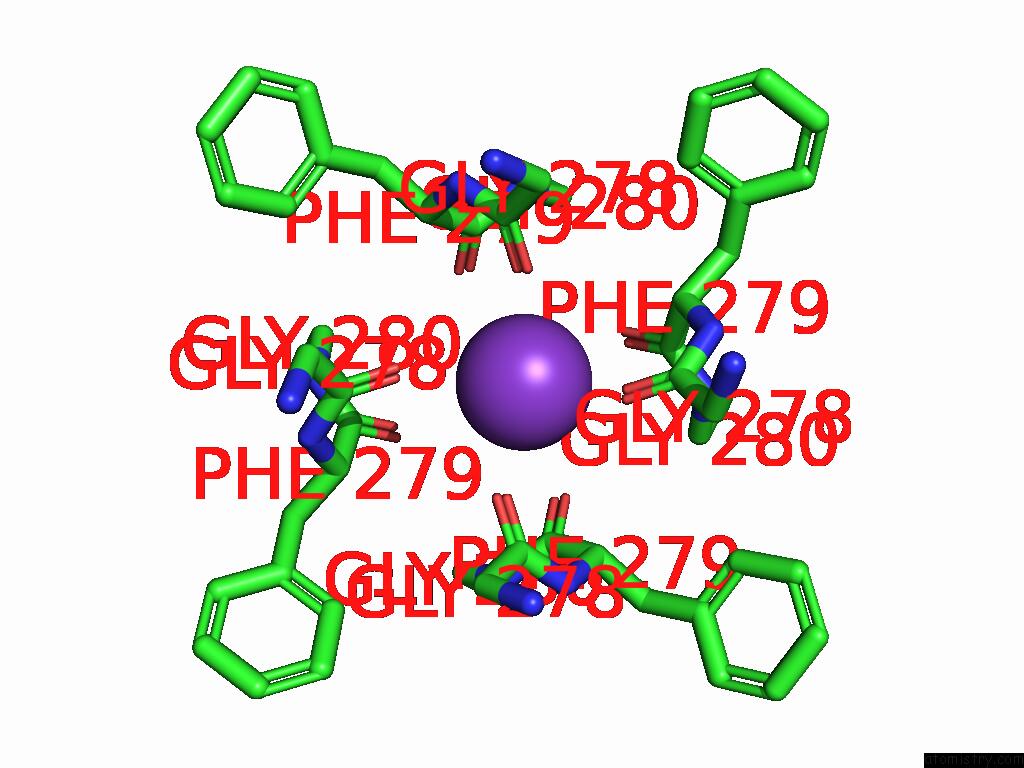
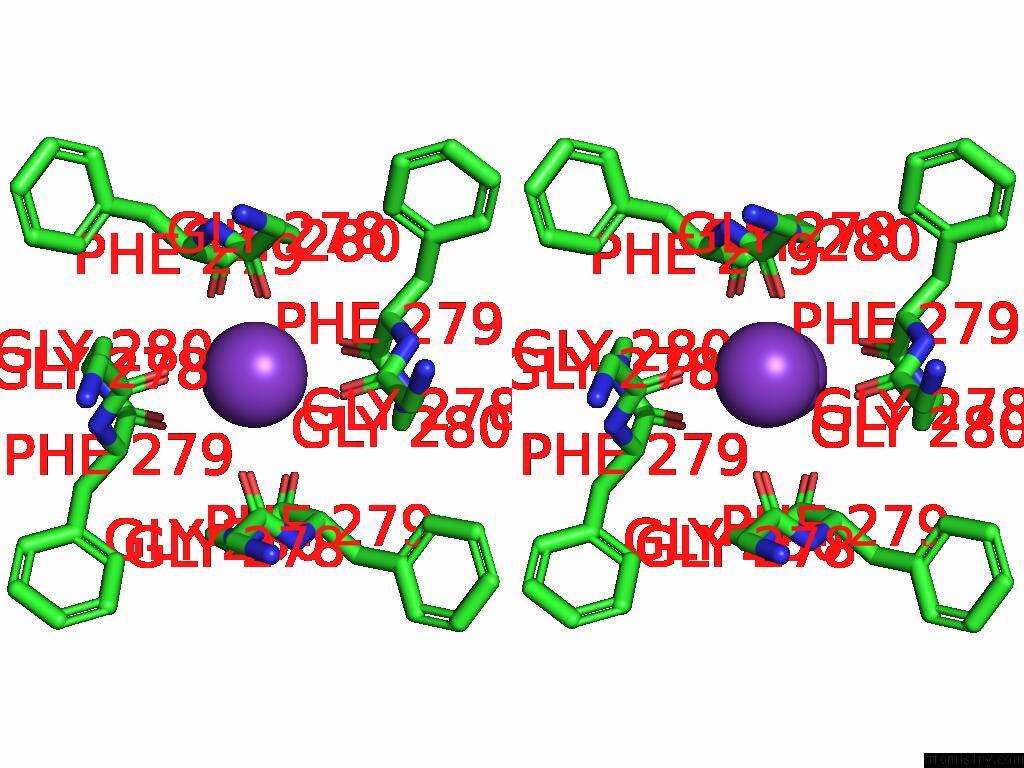
Potassium binding site 3 out of 5 in 9dic
Go back to
Potassium binding site 3 out
of 5 in the Apo Aplysia SLO1 - R196Q
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 3 of Apo Aplysia SLO1 - R196Q within 5.0Å range:
|
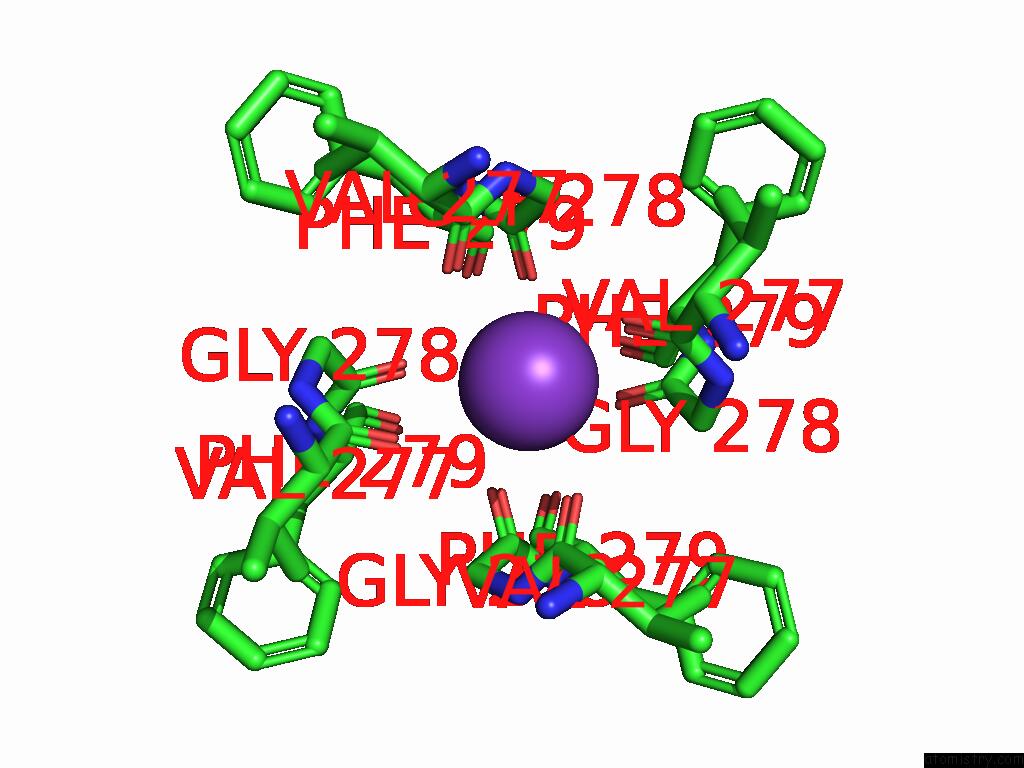
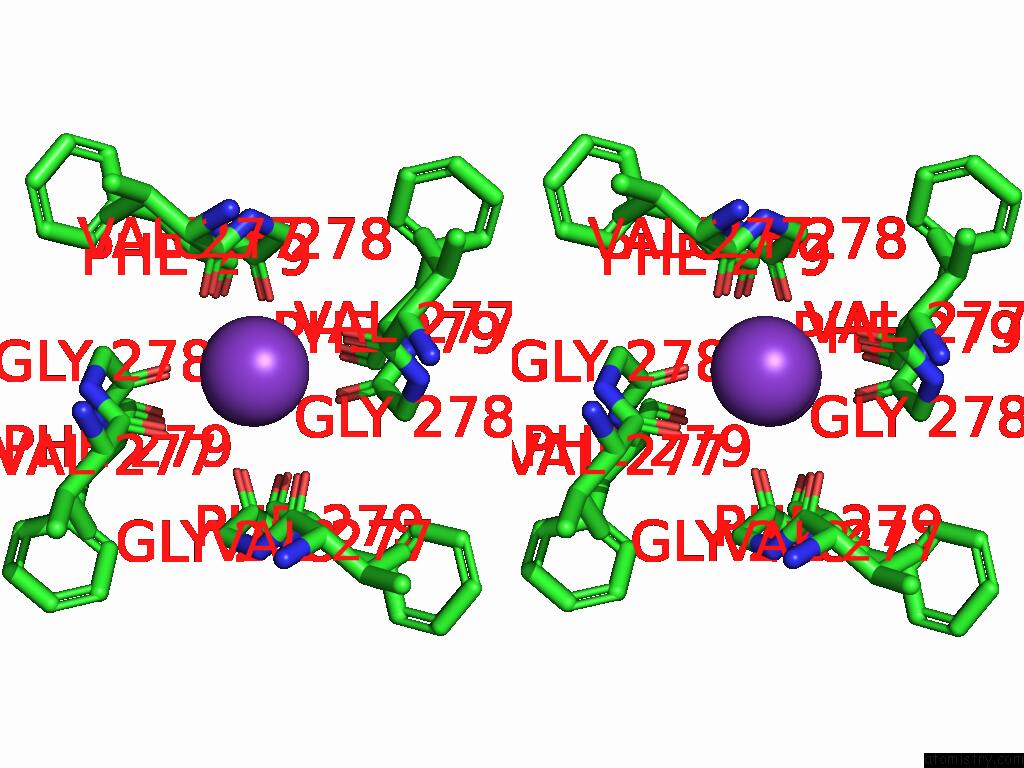
Potassium binding site 4 out of 5 in 9dic
Go back to
Potassium binding site 4 out
of 5 in the Apo Aplysia SLO1 - R196Q
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 4 of Apo Aplysia SLO1 - R196Q within 5.0Å range:
|
Potassium binding site 5 out of 5 in 9dic
Go back to
Potassium binding site 5 out
of 5 in the Apo Aplysia SLO1 - R196Q
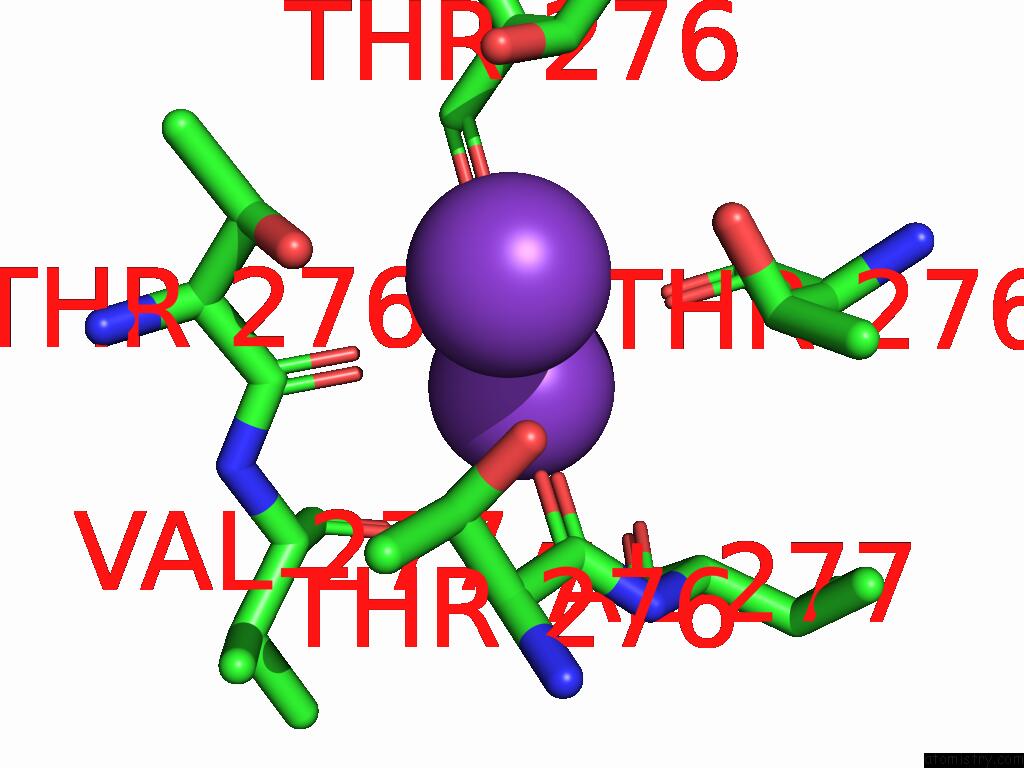
Mono view
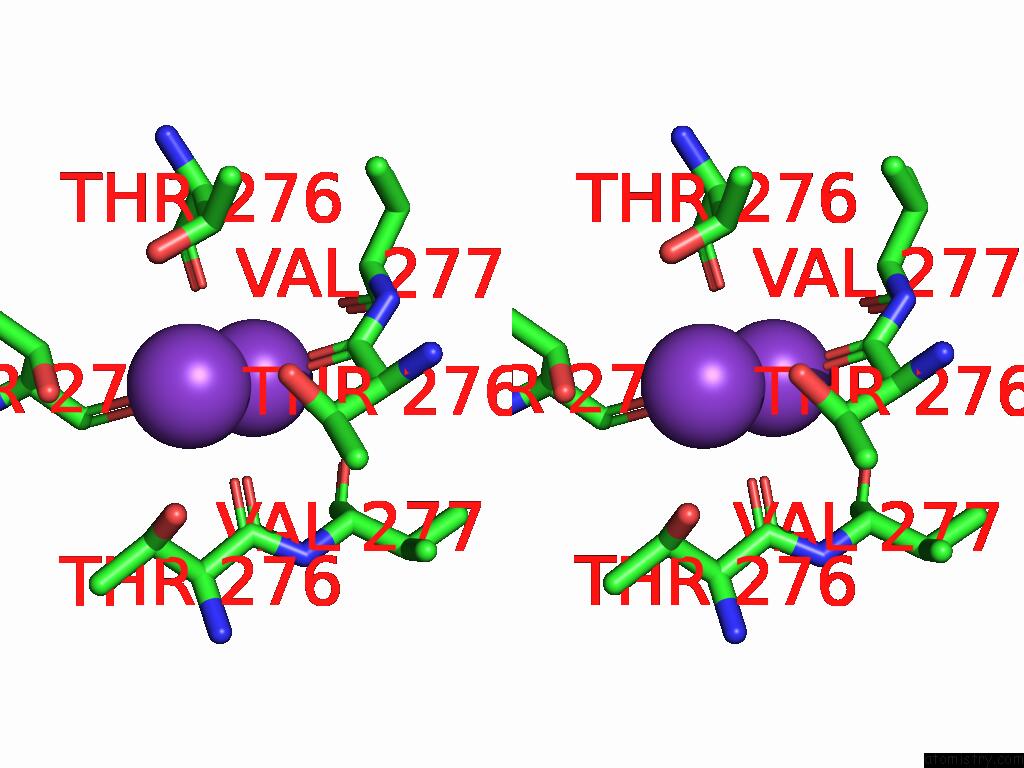
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 5 of Apo Aplysia SLO1 - R196Q within 5.0Å range:
|
Reference:
G.F.Contreras,
R.Shen,
R.Latorre,
E.Perozo.
Structural Basis of Voltage-Dependent Gating in Bk Channels Nat Commun 2025.
ISSN: ESSN 2041-1723
DOI: 10.1038/S41467-025-60639-Y
Page generated: Sat Aug 9 18:40:56 2025
ISSN: ESSN 2041-1723
DOI: 10.1038/S41467-025-60639-Y
Last articles
Mg in 5MMJMg in 5MRA
Mg in 5MTV
Mg in 5MS0
Mg in 5MRU
Mg in 5MQJ
Mg in 5MQW
Mg in 5MQT
Mg in 5MQL
Mg in 5MQ1