Potassium »
PDB 8eqi-8g7l »
8fzu »
Potassium in PDB 8fzu: The Von Willebrand Factor A Domain of Human Capillary Morphogenesis Gene II, Flexibly Fused to the 1TEL Crystallization Chaperone, Thr- Val Linker Variant, Expressed with Sumo Tag
Protein crystallography data
The structure of The Von Willebrand Factor A Domain of Human Capillary Morphogenesis Gene II, Flexibly Fused to the 1TEL Crystallization Chaperone, Thr- Val Linker Variant, Expressed with Sumo Tag, PDB code: 8fzu
was solved by
P.L.Gajjar,
C.M.Litchfield,
M.Callahan,
N.Redd,
T.Doukov,
A.Lebedev,
J.D.Moody,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 71.06 / 1.90 |
| Space group | P 65 |
| Cell size a, b, c (Å), α, β, γ (°) | 164.096, 164.096, 54.401, 90, 90, 120 |
| R / Rfree (%) | 23.8 / 26.6 |
Potassium Binding Sites:
The binding sites of Potassium atom in the The Von Willebrand Factor A Domain of Human Capillary Morphogenesis Gene II, Flexibly Fused to the 1TEL Crystallization Chaperone, Thr- Val Linker Variant, Expressed with Sumo Tag
(pdb code 8fzu). This binding sites where shown within
5.0 Angstroms radius around Potassium atom.
In total only one binding site of Potassium was determined in the The Von Willebrand Factor A Domain of Human Capillary Morphogenesis Gene II, Flexibly Fused to the 1TEL Crystallization Chaperone, Thr- Val Linker Variant, Expressed with Sumo Tag, PDB code: 8fzu:
In total only one binding site of Potassium was determined in the The Von Willebrand Factor A Domain of Human Capillary Morphogenesis Gene II, Flexibly Fused to the 1TEL Crystallization Chaperone, Thr- Val Linker Variant, Expressed with Sumo Tag, PDB code: 8fzu:
Potassium binding site 1 out of 1 in 8fzu
Go back to
Potassium binding site 1 out
of 1 in the The Von Willebrand Factor A Domain of Human Capillary Morphogenesis Gene II, Flexibly Fused to the 1TEL Crystallization Chaperone, Thr- Val Linker Variant, Expressed with Sumo Tag
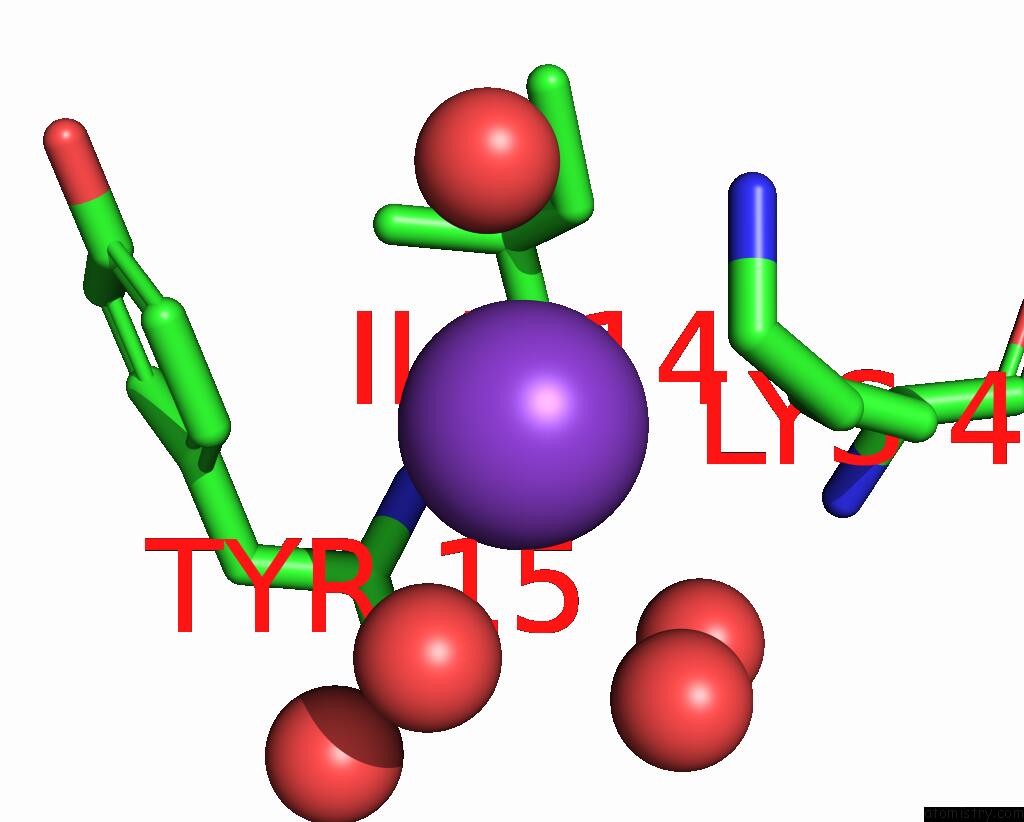
Mono view
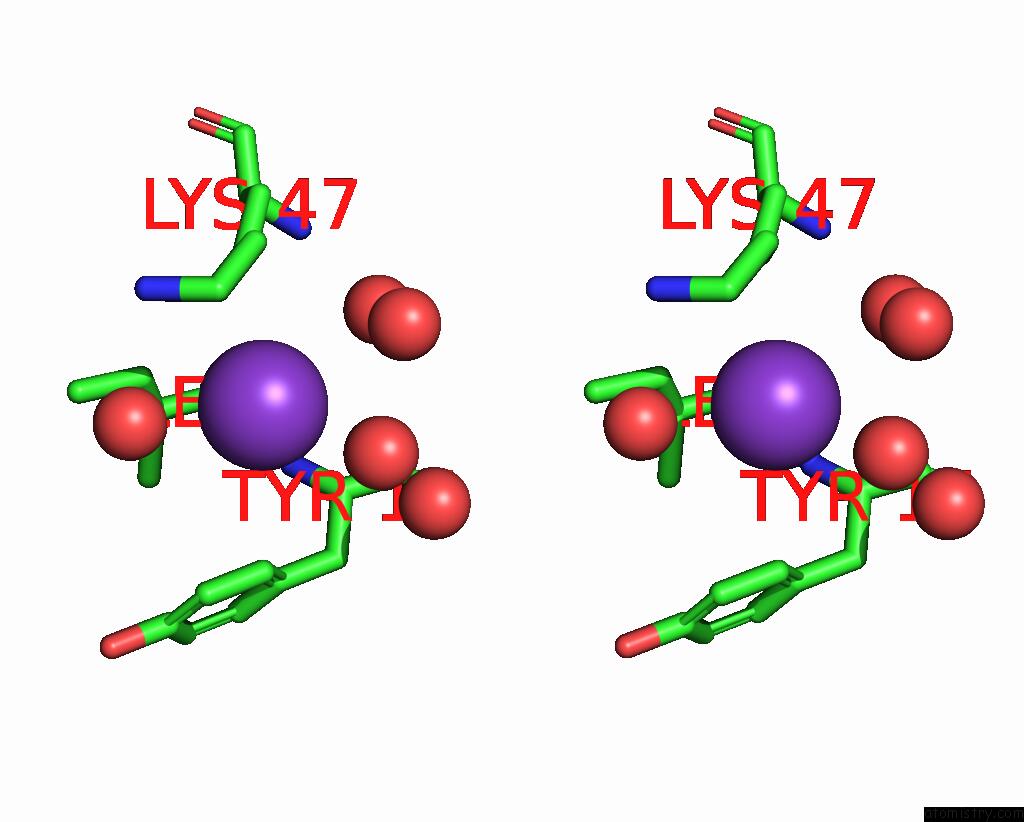
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 1 of The Von Willebrand Factor A Domain of Human Capillary Morphogenesis Gene II, Flexibly Fused to the 1TEL Crystallization Chaperone, Thr- Val Linker Variant, Expressed with Sumo Tag within 5.0Å range:
|
Reference:
P.L.Gajjar,
M.J.P.Romo,
C.M.Litchfield,
M.Callahan,
N.Redd,
S.Nawarathnage,
S.Soleimani,
J.Averett,
E.Wilson,
A.Lewis,
C.Stewart,
Y.J.Tseng,
T.Doukov,
A.Lebedev,
J.D.Moody.
Decreasing the Flexibility of the Telsam-Target Protein Linker and Omitting the Cleavable Fusion Tag Improves Crystal Order and Diffraction Limits. Biorxiv 2023.
ISSN: ISSN 2692-8205
PubMed: 37293010
DOI: 10.1101/2023.05.12.540586
Page generated: Mon Aug 12 23:44:34 2024
ISSN: ISSN 2692-8205
PubMed: 37293010
DOI: 10.1101/2023.05.12.540586
Last articles
Zn in 9JYWZn in 9IR4
Zn in 9IR3
Zn in 9GMX
Zn in 9GMW
Zn in 9JEJ
Zn in 9ERF
Zn in 9ERE
Zn in 9EGV
Zn in 9EGW