Potassium »
PDB 7pxh-7r2w »
7qdn »
Potassium in PDB 7qdn: Structure of Human Liver Pyruvate Kinase From Which the B Domain Has Been Deleted
Enzymatic activity of Structure of Human Liver Pyruvate Kinase From Which the B Domain Has Been Deleted
All present enzymatic activity of Structure of Human Liver Pyruvate Kinase From Which the B Domain Has Been Deleted:
2.7.1.40;
2.7.1.40;
Protein crystallography data
The structure of Structure of Human Liver Pyruvate Kinase From Which the B Domain Has Been Deleted, PDB code: 7qdn
was solved by
A.Lulla,
M.Hyvonen,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 103.69 / 1.70 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 207.448, 112.687, 188.333, 90, 91.36, 90 |
| R / Rfree (%) | 20.5 / 21.7 |
Other elements in 7qdn:
The structure of Structure of Human Liver Pyruvate Kinase From Which the B Domain Has Been Deleted also contains other interesting chemical elements:
| Sodium | (Na) | 6 atoms |
| Magnesium | (Mg) | 8 atoms |
Potassium Binding Sites:
The binding sites of Potassium atom in the Structure of Human Liver Pyruvate Kinase From Which the B Domain Has Been Deleted
(pdb code 7qdn). This binding sites where shown within
5.0 Angstroms radius around Potassium atom.
In total 8 binding sites of Potassium where determined in the Structure of Human Liver Pyruvate Kinase From Which the B Domain Has Been Deleted, PDB code: 7qdn:
Jump to Potassium binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
In total 8 binding sites of Potassium where determined in the Structure of Human Liver Pyruvate Kinase From Which the B Domain Has Been Deleted, PDB code: 7qdn:
Jump to Potassium binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
Potassium binding site 1 out of 8 in 7qdn
Go back to
Potassium binding site 1 out
of 8 in the Structure of Human Liver Pyruvate Kinase From Which the B Domain Has Been Deleted
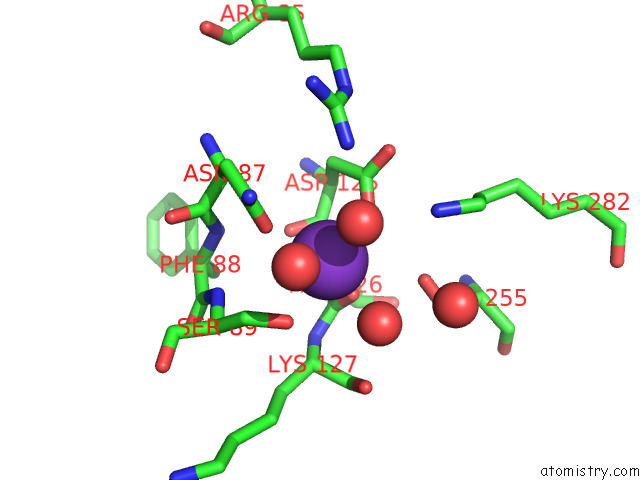
Mono view
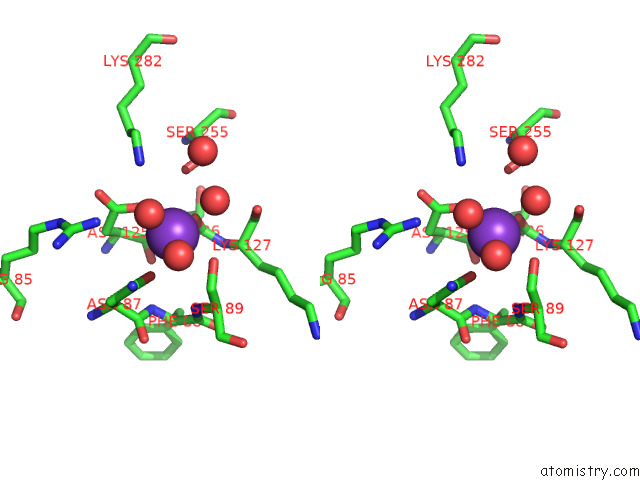
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 1 of Structure of Human Liver Pyruvate Kinase From Which the B Domain Has Been Deleted within 5.0Å range:
|
Potassium binding site 2 out of 8 in 7qdn
Go back to
Potassium binding site 2 out
of 8 in the Structure of Human Liver Pyruvate Kinase From Which the B Domain Has Been Deleted
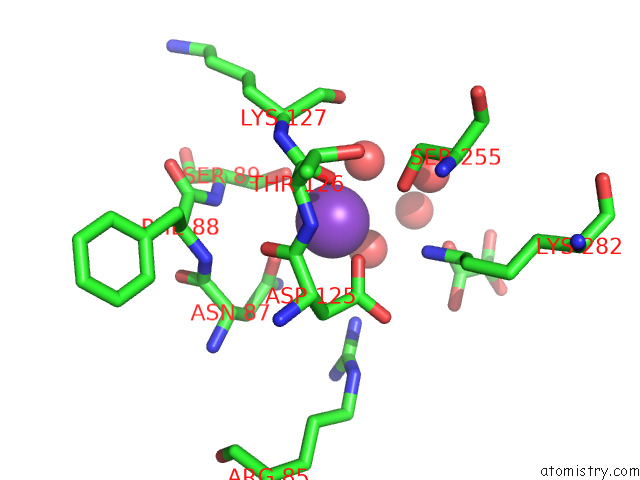
Mono view
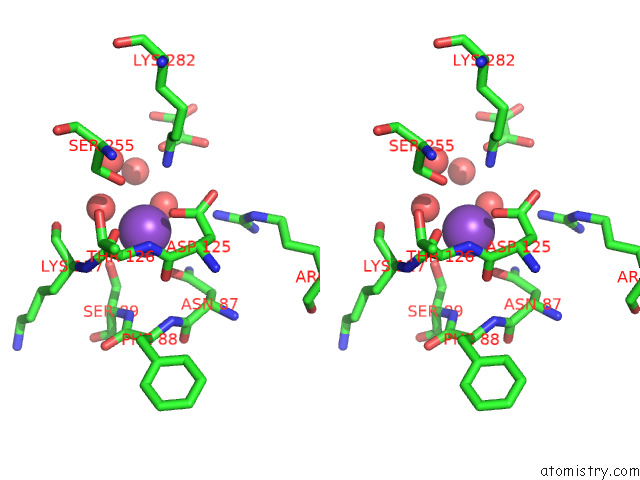
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 2 of Structure of Human Liver Pyruvate Kinase From Which the B Domain Has Been Deleted within 5.0Å range:
|
Potassium binding site 3 out of 8 in 7qdn
Go back to
Potassium binding site 3 out
of 8 in the Structure of Human Liver Pyruvate Kinase From Which the B Domain Has Been Deleted
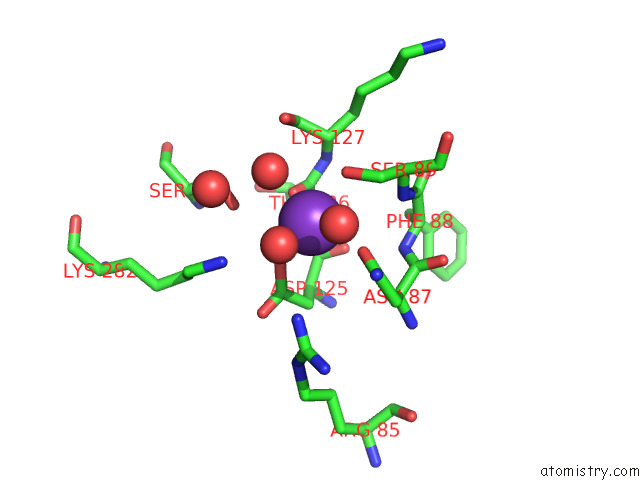
Mono view
Stereo pair view

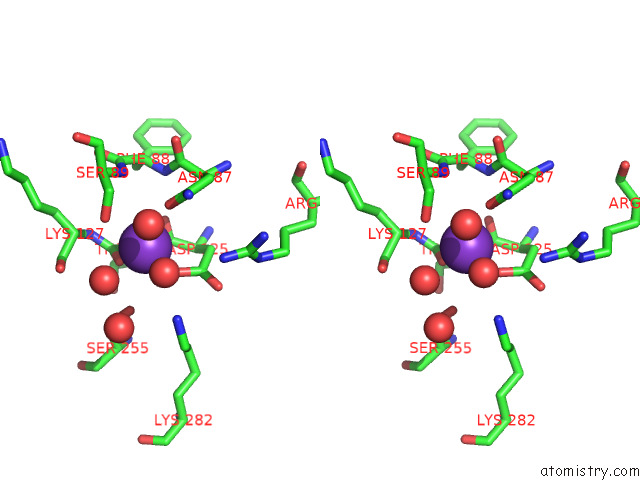
Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 3 of Structure of Human Liver Pyruvate Kinase From Which the B Domain Has Been Deleted within 5.0Å range:
|
Potassium binding site 4 out of 8 in 7qdn
Go back to
Potassium binding site 4 out
of 8 in the Structure of Human Liver Pyruvate Kinase From Which the B Domain Has Been Deleted
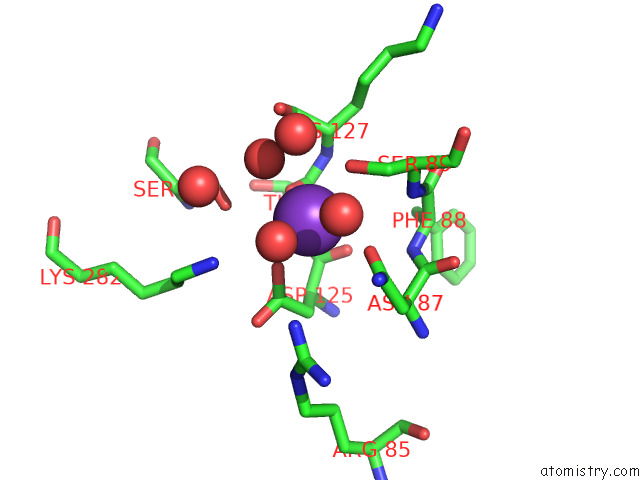
Mono view
Stereo pair view

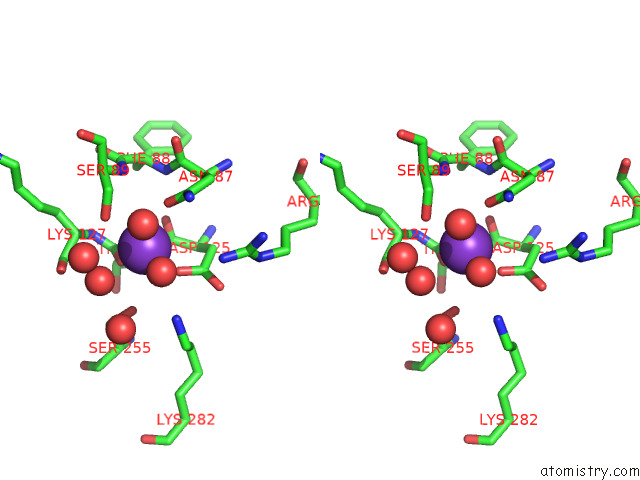
Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 4 of Structure of Human Liver Pyruvate Kinase From Which the B Domain Has Been Deleted within 5.0Å range:
|
Potassium binding site 5 out of 8 in 7qdn
Go back to
Potassium binding site 5 out
of 8 in the Structure of Human Liver Pyruvate Kinase From Which the B Domain Has Been Deleted
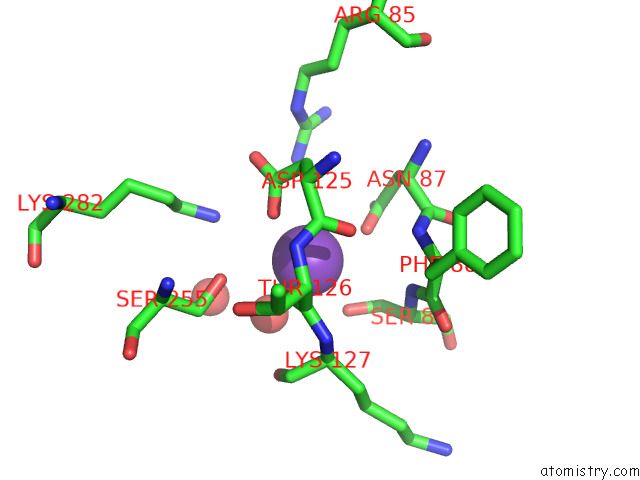
Mono view
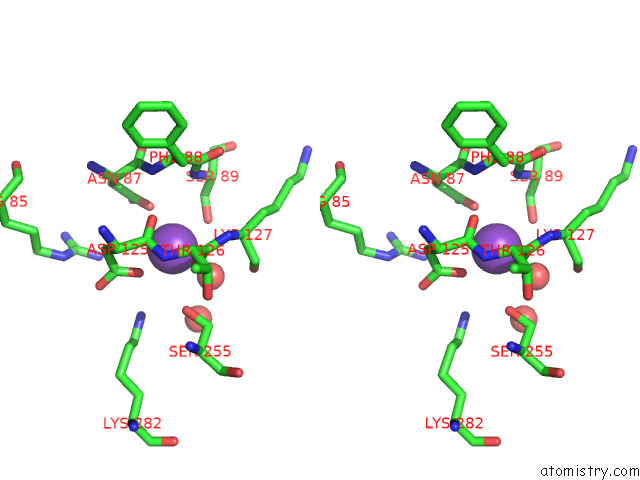
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 5 of Structure of Human Liver Pyruvate Kinase From Which the B Domain Has Been Deleted within 5.0Å range:
|
Potassium binding site 6 out of 8 in 7qdn
Go back to
Potassium binding site 6 out
of 8 in the Structure of Human Liver Pyruvate Kinase From Which the B Domain Has Been Deleted
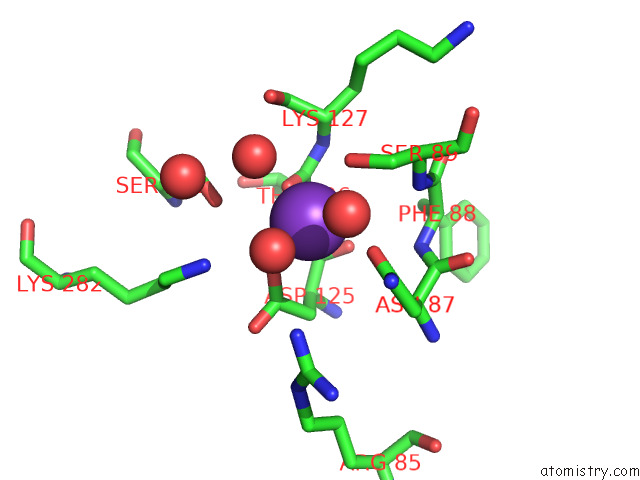
Mono view
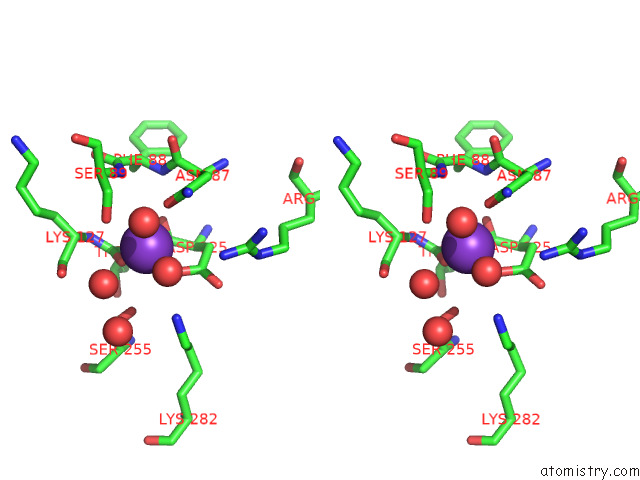
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 6 of Structure of Human Liver Pyruvate Kinase From Which the B Domain Has Been Deleted within 5.0Å range:
|
Potassium binding site 7 out of 8 in 7qdn
Go back to
Potassium binding site 7 out
of 8 in the Structure of Human Liver Pyruvate Kinase From Which the B Domain Has Been Deleted
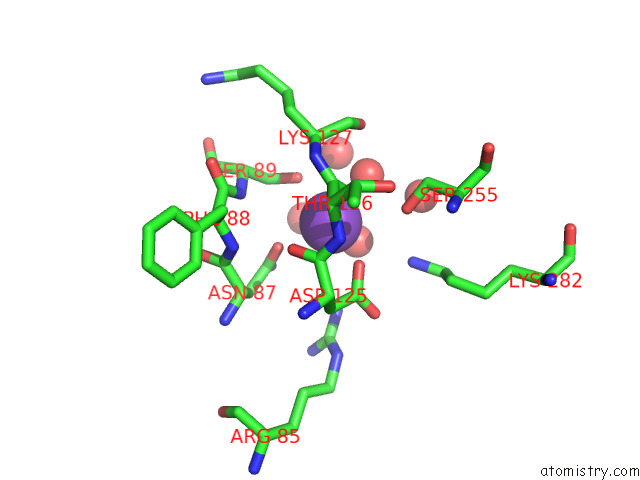
Mono view
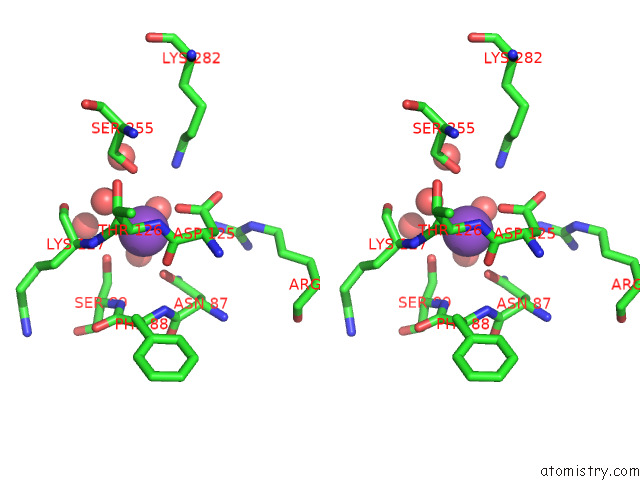
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 7 of Structure of Human Liver Pyruvate Kinase From Which the B Domain Has Been Deleted within 5.0Å range:
|
Potassium binding site 8 out of 8 in 7qdn
Go back to
Potassium binding site 8 out
of 8 in the Structure of Human Liver Pyruvate Kinase From Which the B Domain Has Been Deleted
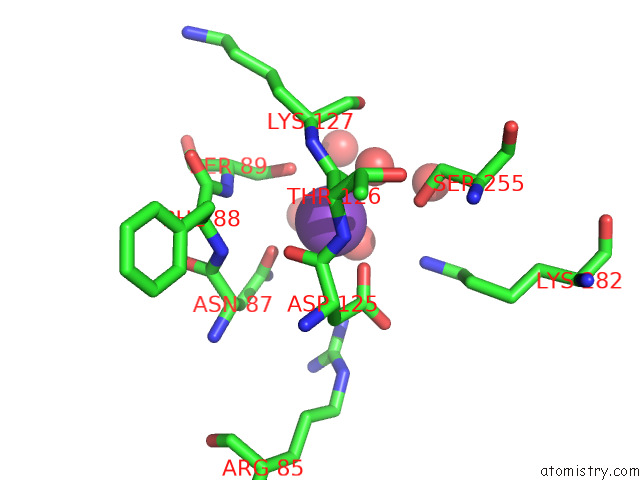
Mono view
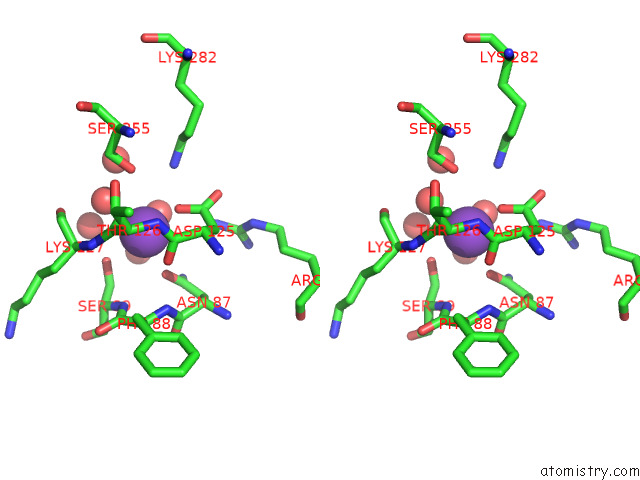
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 8 of Structure of Human Liver Pyruvate Kinase From Which the B Domain Has Been Deleted within 5.0Å range:
|
Reference:
A.Nain-Perez,
A.Foller Fuchtbauer,
L.Haversen,
A.Lulla,
C.Gao,
J.Matic,
L.Monjas,
A.Rodriguez,
P.Brear,
W.Kim,
M.Hyvonen,
J.Boren,
A.Mardinoglu,
M.Uhlen,
M.Grotli.
Anthraquinone Derivatives As Adp-Competitive Inhibitors of Liver Pyruvate Kinase. Eur.J.Med.Chem. V. 234 14270 2022.
ISSN: ISSN 0223-5234
PubMed: 35290845
DOI: 10.1016/J.EJMECH.2022.114270
Page generated: Mon Aug 12 20:27:49 2024
ISSN: ISSN 0223-5234
PubMed: 35290845
DOI: 10.1016/J.EJMECH.2022.114270
Last articles
Zn in 9JYWZn in 9IR4
Zn in 9IR3
Zn in 9GMX
Zn in 9GMW
Zn in 9JEJ
Zn in 9ERF
Zn in 9ERE
Zn in 9EGV
Zn in 9EGW