Potassium »
PDB 7kxa-7n9k »
7n9k »
Potassium in PDB 7n9k: KIRBAC3.1 L124M Mutant
Protein crystallography data
The structure of KIRBAC3.1 L124M Mutant, PDB code: 7n9k
was solved by
T.A.Black,
J.M.Gulbis,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 42.08 / 2.72 |
| Space group | P 4 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 106.577, 106.577, 89.597, 90, 90, 90 |
| R / Rfree (%) | 21.8 / 25 |
Potassium Binding Sites:
The binding sites of Potassium atom in the KIRBAC3.1 L124M Mutant
(pdb code 7n9k). This binding sites where shown within
5.0 Angstroms radius around Potassium atom.
In total 7 binding sites of Potassium where determined in the KIRBAC3.1 L124M Mutant, PDB code: 7n9k:
Jump to Potassium binding site number: 1; 2; 3; 4; 5; 6; 7;
In total 7 binding sites of Potassium where determined in the KIRBAC3.1 L124M Mutant, PDB code: 7n9k:
Jump to Potassium binding site number: 1; 2; 3; 4; 5; 6; 7;
Potassium binding site 1 out of 7 in 7n9k
Go back to
Potassium binding site 1 out
of 7 in the KIRBAC3.1 L124M Mutant
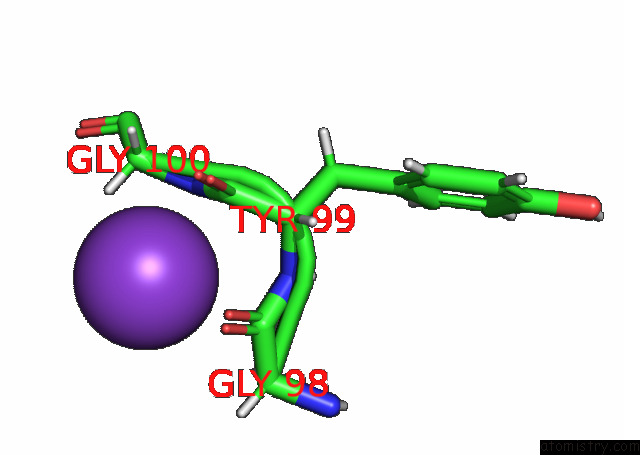
Mono view
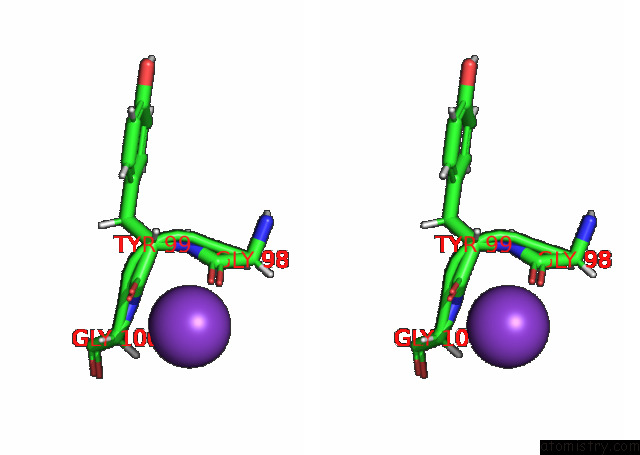
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 1 of KIRBAC3.1 L124M Mutant within 5.0Å range:
|
Potassium binding site 2 out of 7 in 7n9k
Go back to
Potassium binding site 2 out
of 7 in the KIRBAC3.1 L124M Mutant
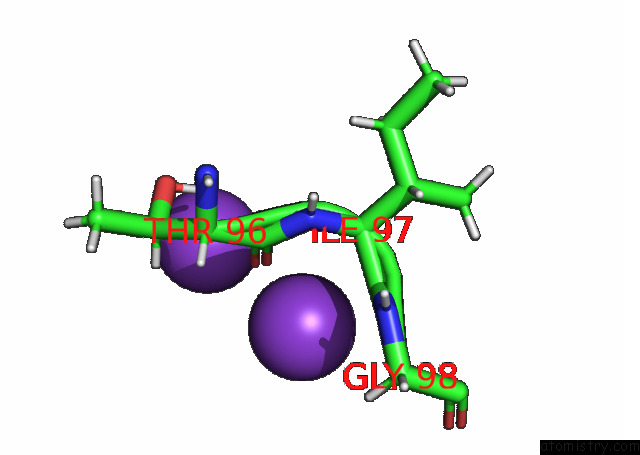
Mono view
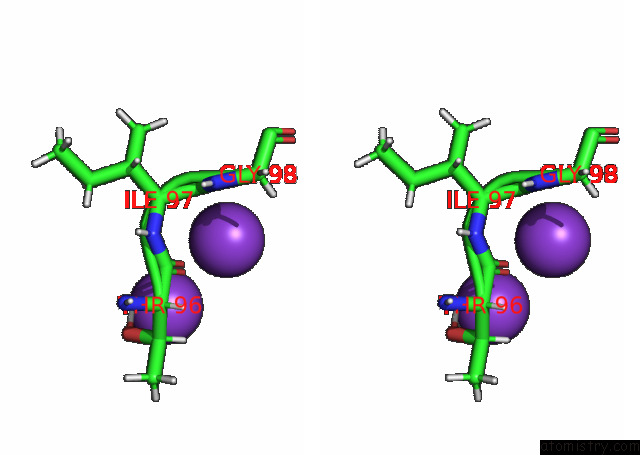
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 2 of KIRBAC3.1 L124M Mutant within 5.0Å range:
|
Potassium binding site 3 out of 7 in 7n9k
Go back to
Potassium binding site 3 out
of 7 in the KIRBAC3.1 L124M Mutant
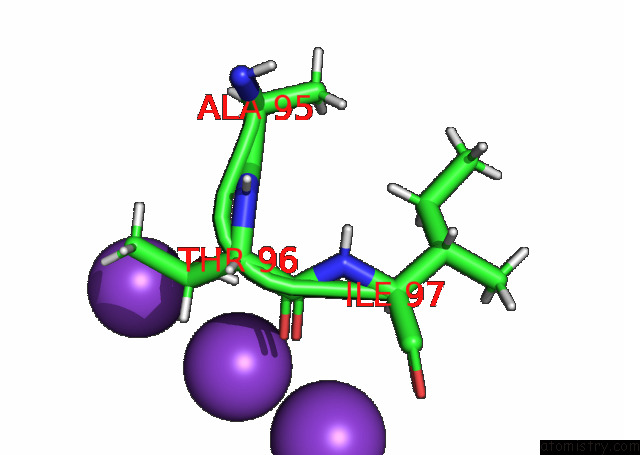
Mono view
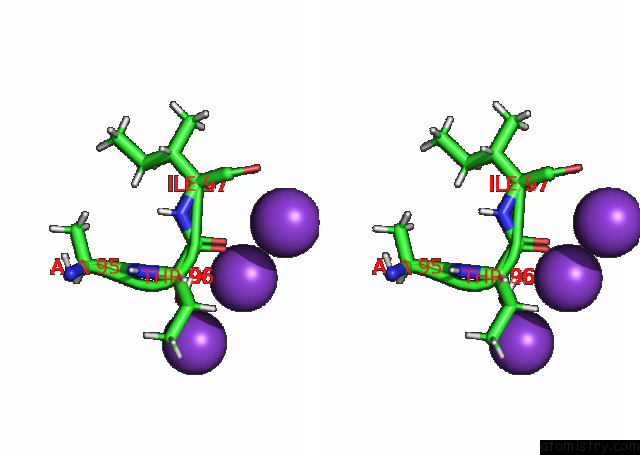
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 3 of KIRBAC3.1 L124M Mutant within 5.0Å range:
|
Potassium binding site 4 out of 7 in 7n9k
Go back to
Potassium binding site 4 out
of 7 in the KIRBAC3.1 L124M Mutant
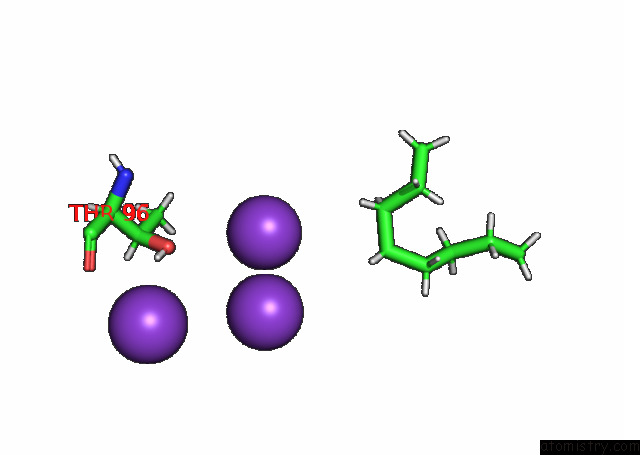
Mono view
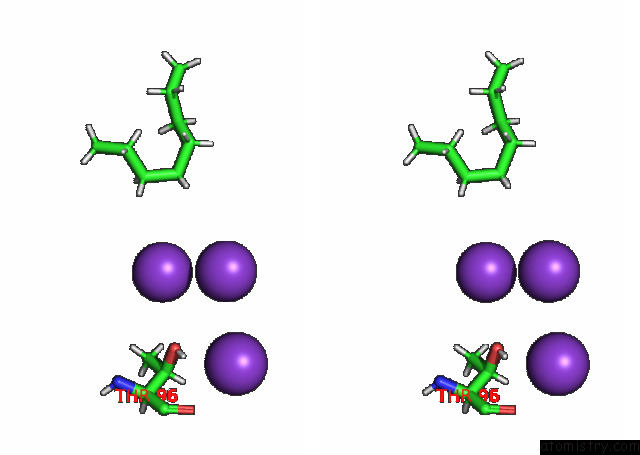
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 4 of KIRBAC3.1 L124M Mutant within 5.0Å range:
|
Potassium binding site 5 out of 7 in 7n9k
Go back to
Potassium binding site 5 out
of 7 in the KIRBAC3.1 L124M Mutant
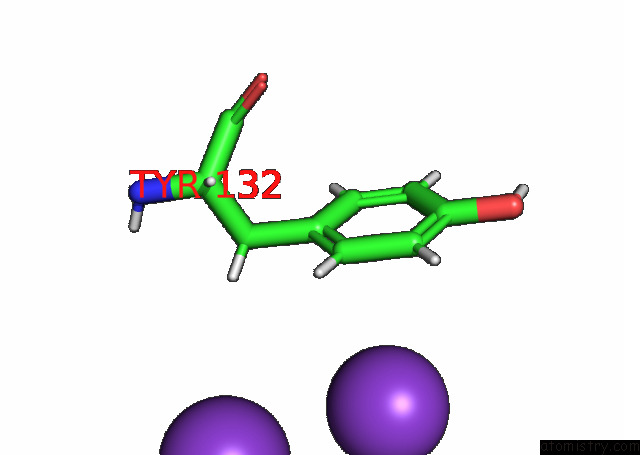
Mono view
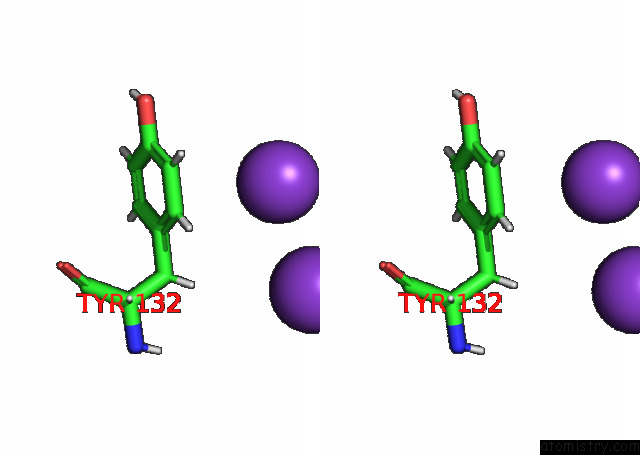
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 5 of KIRBAC3.1 L124M Mutant within 5.0Å range:
|
Potassium binding site 6 out of 7 in 7n9k
Go back to
Potassium binding site 6 out
of 7 in the KIRBAC3.1 L124M Mutant
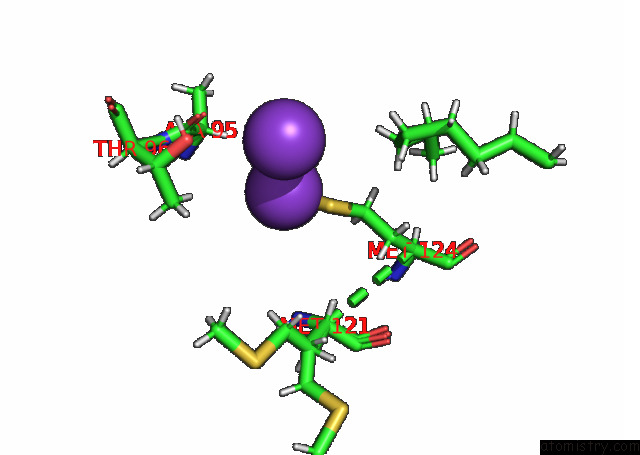
Mono view
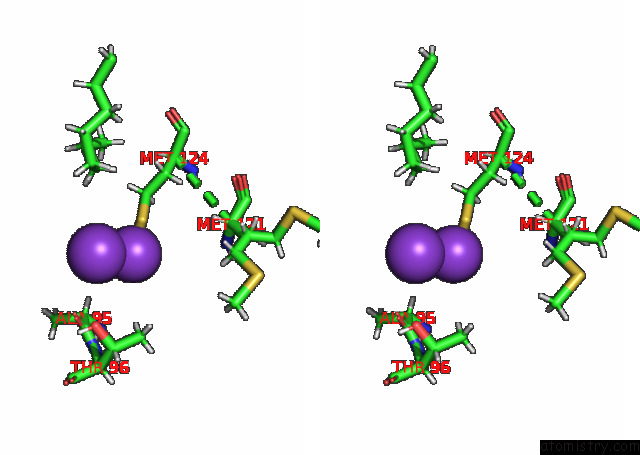
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 6 of KIRBAC3.1 L124M Mutant within 5.0Å range:
|
Potassium binding site 7 out of 7 in 7n9k
Go back to
Potassium binding site 7 out
of 7 in the KIRBAC3.1 L124M Mutant
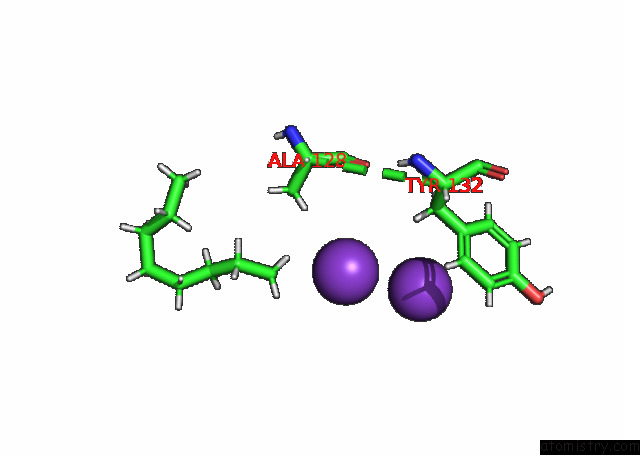
Mono view
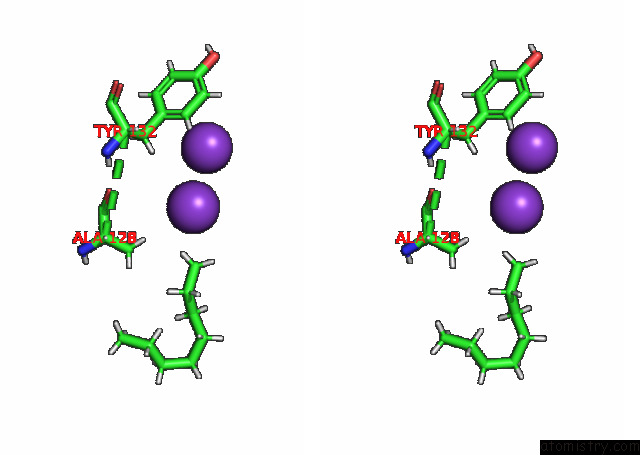
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 7 of KIRBAC3.1 L124M Mutant within 5.0Å range:
|
Reference:
J.M.Gulbis,
K.A.Black.
Ion Currents Through Kir Potassium Channels Are Gated By Anionic Lipids. Nat Commun 2021.
ISSN: ESSN 2041-1723
Page generated: Sat Aug 9 13:42:06 2025
ISSN: ESSN 2041-1723
Last articles
Mg in 2IS6Mg in 2ISI
Mg in 2ITN
Mg in 2ISP
Mg in 2ISO
Mg in 2IOU
Mg in 2IS4
Mg in 2IOH
Mg in 2IQQ
Mg in 2IQ1