Potassium »
PDB 6z7v-7adi »
7adi »
Potassium in PDB 7adi: KIRBAC3.1 W46R: Role of A Highly Conserved Tryptophan at the Membrane- Water Interface of Kir Channel
Protein crystallography data
The structure of KIRBAC3.1 W46R: Role of A Highly Conserved Tryptophan at the Membrane- Water Interface of Kir Channel, PDB code: 7adi
was solved by
C.Venien-Bryan,
C.Fagnen,
R.De Zorzi,
L.Bannwarth,
I.Oubella,
A.Haouz,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 48.02 / 2.80 |
| Space group | P 21 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 106.77, 113.98, 89.18, 90, 90, 90 |
| R / Rfree (%) | 22.2 / 28.7 |
Other elements in 7adi:
The structure of KIRBAC3.1 W46R: Role of A Highly Conserved Tryptophan at the Membrane- Water Interface of Kir Channel also contains other interesting chemical elements:
| Magnesium | (Mg) | 1 atom |
Potassium Binding Sites:
The binding sites of Potassium atom in the KIRBAC3.1 W46R: Role of A Highly Conserved Tryptophan at the Membrane- Water Interface of Kir Channel
(pdb code 7adi). This binding sites where shown within
5.0 Angstroms radius around Potassium atom.
In total 3 binding sites of Potassium where determined in the KIRBAC3.1 W46R: Role of A Highly Conserved Tryptophan at the Membrane- Water Interface of Kir Channel, PDB code: 7adi:
Jump to Potassium binding site number: 1; 2; 3;
In total 3 binding sites of Potassium where determined in the KIRBAC3.1 W46R: Role of A Highly Conserved Tryptophan at the Membrane- Water Interface of Kir Channel, PDB code: 7adi:
Jump to Potassium binding site number: 1; 2; 3;
Potassium binding site 1 out of 3 in 7adi
Go back to
Potassium binding site 1 out
of 3 in the KIRBAC3.1 W46R: Role of A Highly Conserved Tryptophan at the Membrane- Water Interface of Kir Channel
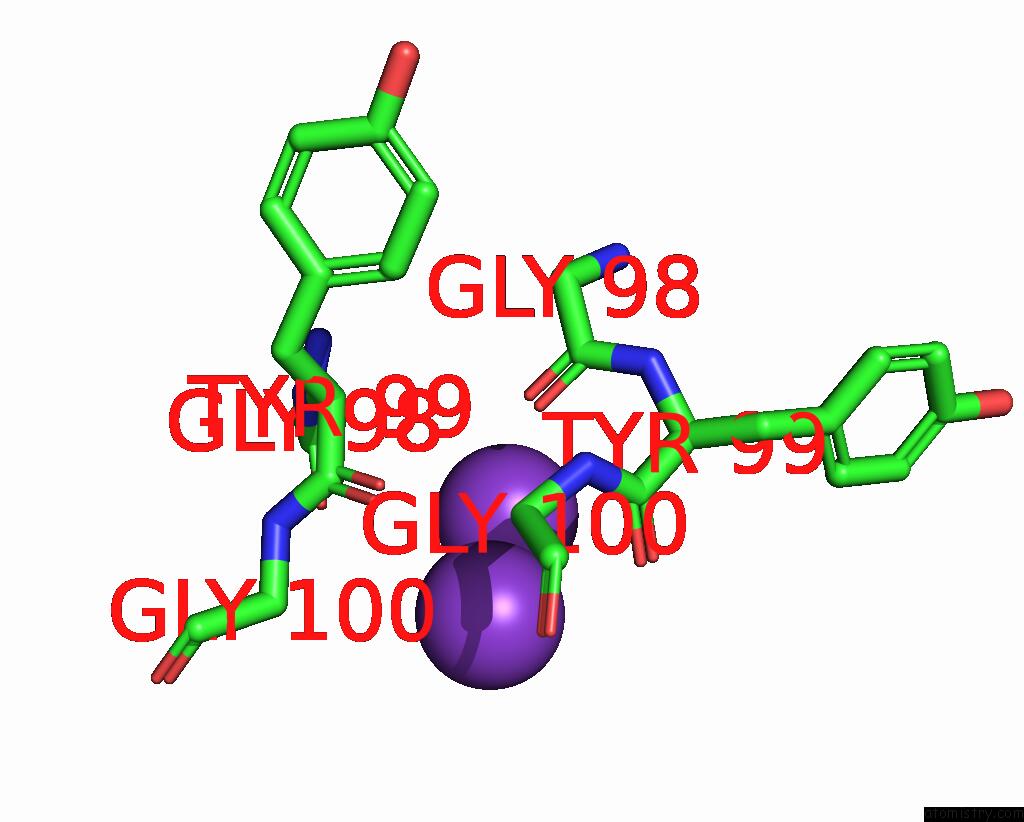
Mono view
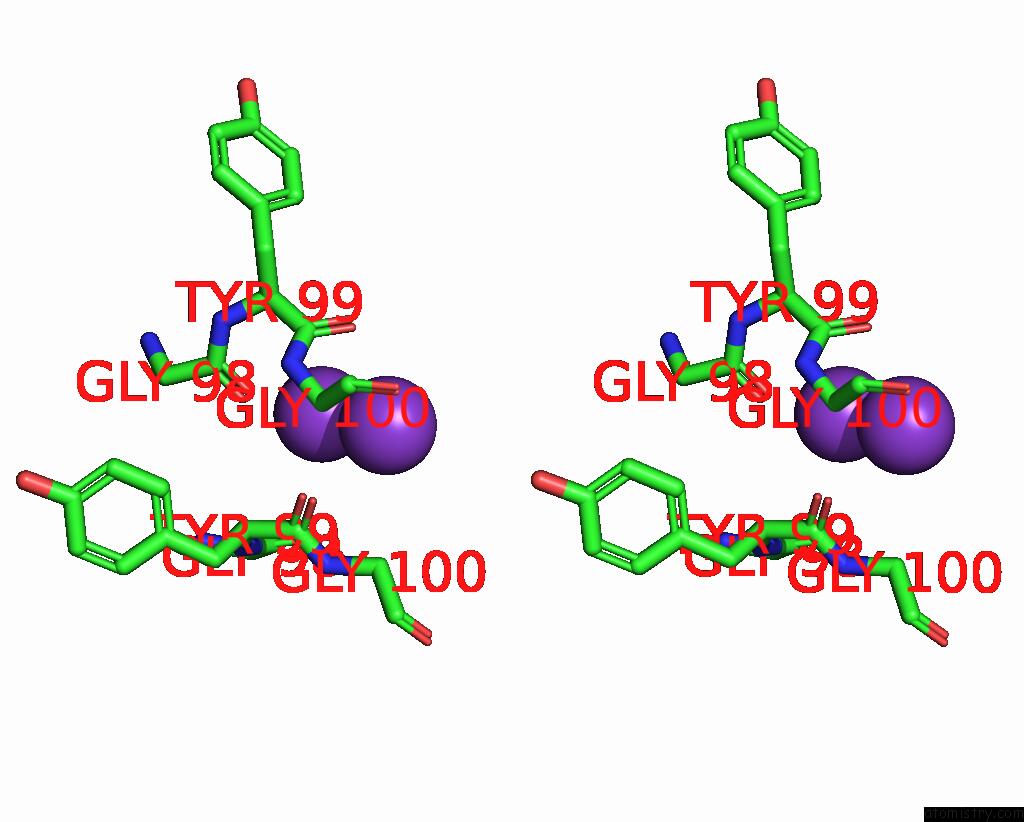
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 1 of KIRBAC3.1 W46R: Role of A Highly Conserved Tryptophan at the Membrane- Water Interface of Kir Channel within 5.0Å range:
|
Potassium binding site 2 out of 3 in 7adi
Go back to
Potassium binding site 2 out
of 3 in the KIRBAC3.1 W46R: Role of A Highly Conserved Tryptophan at the Membrane- Water Interface of Kir Channel
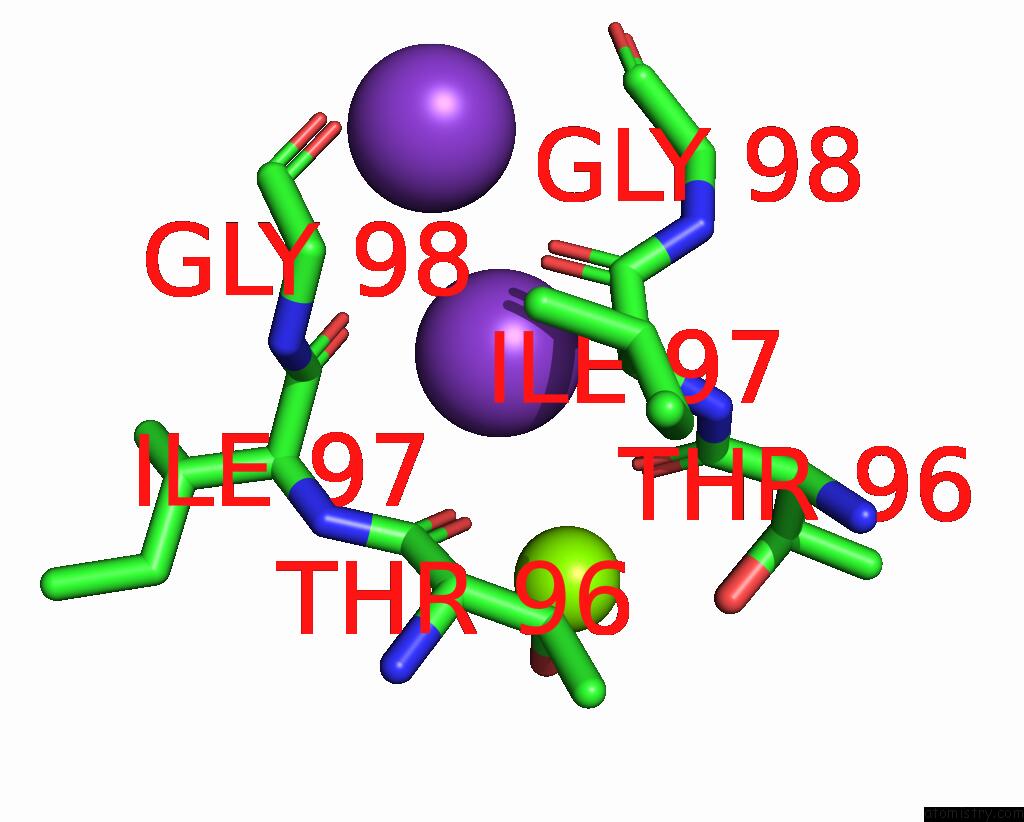
Mono view
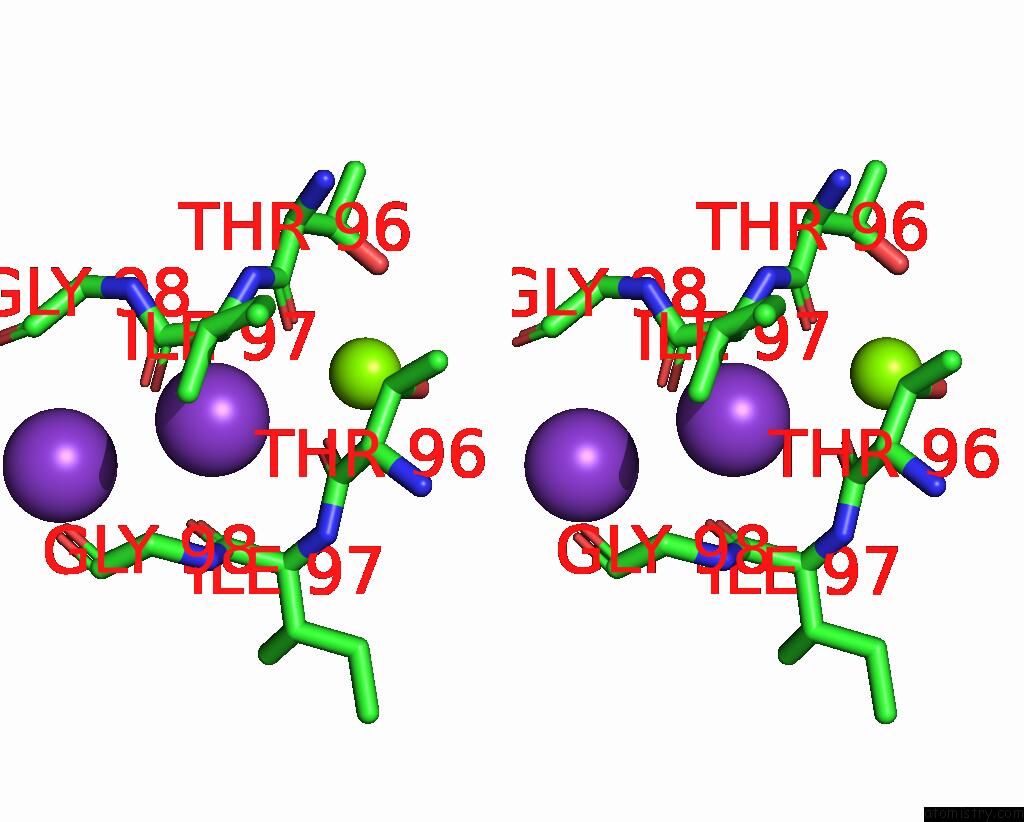
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 2 of KIRBAC3.1 W46R: Role of A Highly Conserved Tryptophan at the Membrane- Water Interface of Kir Channel within 5.0Å range:
|
Potassium binding site 3 out of 3 in 7adi
Go back to
Potassium binding site 3 out
of 3 in the KIRBAC3.1 W46R: Role of A Highly Conserved Tryptophan at the Membrane- Water Interface of Kir Channel
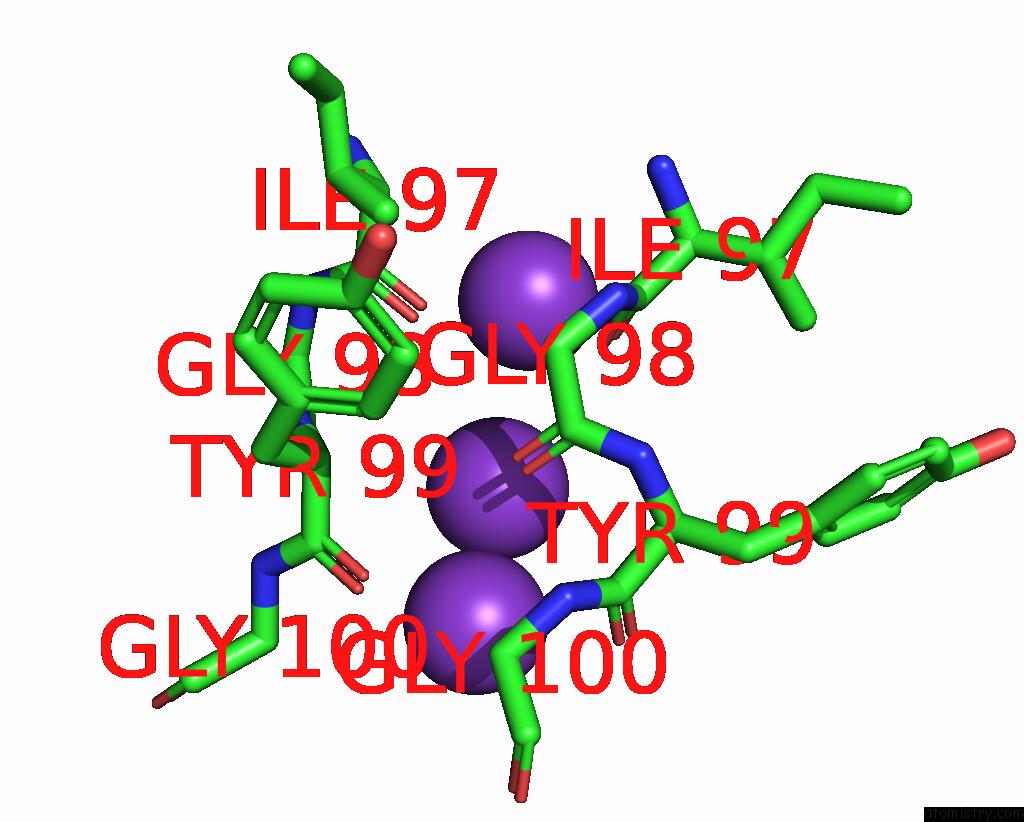
Mono view
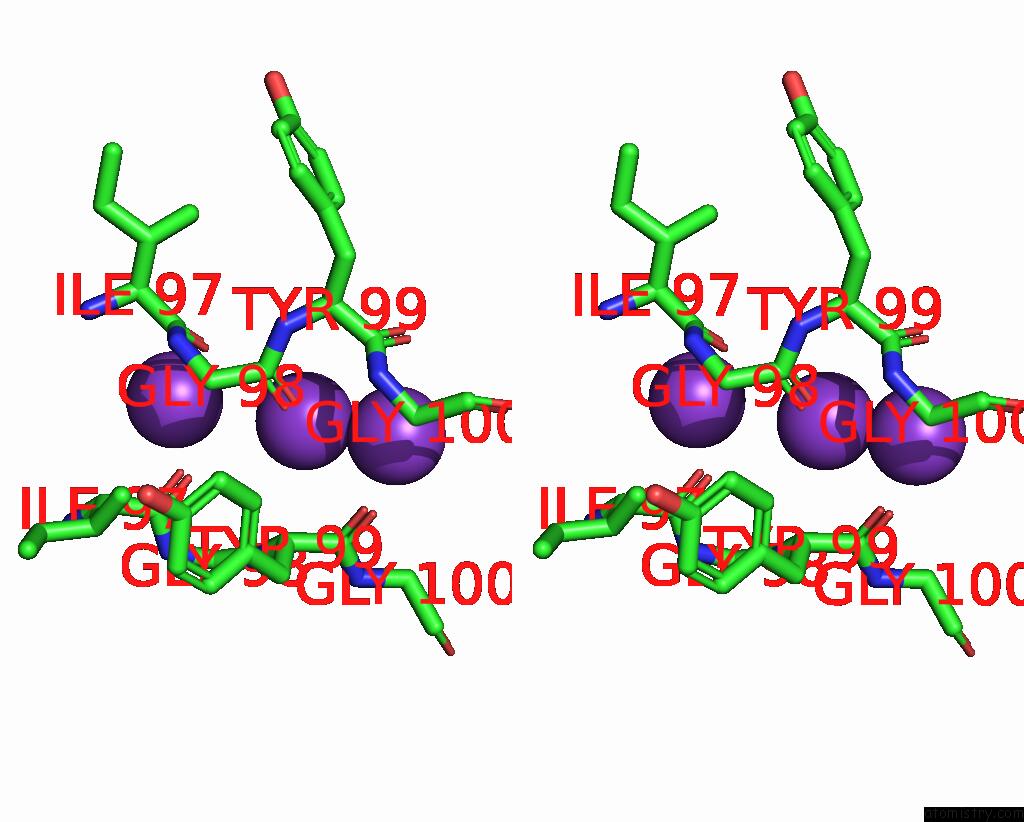
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 3 of KIRBAC3.1 W46R: Role of A Highly Conserved Tryptophan at the Membrane- Water Interface of Kir Channel within 5.0Å range:
|
Reference:
C.Fagnen,
L.Bannwarth,
I.Oubella,
D.Zuniga,
A.Haouz,
E.Forest,
R.Scala,
S.Bendahhou,
R.De Zorzi,
D.Perahia,
C.Venien-Bryan.
Integrative Study of the Structural and Dynamical Properties of A KIRBAC3.1 Mutant: Functional Implication of A Highly Conserved Tryptophan in the Transmembrane Domain. Int J Mol Sci V. 23 2021.
ISSN: ESSN 1422-0067
PubMed: 35008764
DOI: 10.3390/IJMS23010335
Page generated: Sat Aug 9 12:55:36 2025
ISSN: ESSN 1422-0067
PubMed: 35008764
DOI: 10.3390/IJMS23010335
Last articles
Mg in 2PRNMg in 2PPQ
Mg in 2PPB
Mg in 2PLY
Mg in 2PNQ
Mg in 2PP3
Mg in 2PP1
Mg in 2PMQ
Mg in 2PN3
Mg in 2PN6