Potassium »
PDB 6qml-6rv4 »
6rkc »
Potassium in PDB 6rkc: Inter-Dimeric Interface Controls Function and Stability of S- Methionine Adenosyltransferase From U. Urealiticum
Enzymatic activity of Inter-Dimeric Interface Controls Function and Stability of S- Methionine Adenosyltransferase From U. Urealiticum
All present enzymatic activity of Inter-Dimeric Interface Controls Function and Stability of S- Methionine Adenosyltransferase From U. Urealiticum:
2.5.1.6;
2.5.1.6;
Protein crystallography data
The structure of Inter-Dimeric Interface Controls Function and Stability of S- Methionine Adenosyltransferase From U. Urealiticum, PDB code: 6rkc
was solved by
A.Shahar,
R.Zarivach,
S.Bershtein,
D.Kleiner,
F.Shmulevich,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 47.78 / 2.56 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 142.372, 79.467, 143.787, 90.00, 105.11, 90.00 |
| R / Rfree (%) | 26 / 28.4 |
Other elements in 6rkc:
The structure of Inter-Dimeric Interface Controls Function and Stability of S- Methionine Adenosyltransferase From U. Urealiticum also contains other interesting chemical elements:
| Magnesium | (Mg) | 16 atoms |
Potassium Binding Sites:
The binding sites of Potassium atom in the Inter-Dimeric Interface Controls Function and Stability of S- Methionine Adenosyltransferase From U. Urealiticum
(pdb code 6rkc). This binding sites where shown within
5.0 Angstroms radius around Potassium atom.
In total 7 binding sites of Potassium where determined in the Inter-Dimeric Interface Controls Function and Stability of S- Methionine Adenosyltransferase From U. Urealiticum, PDB code: 6rkc:
Jump to Potassium binding site number: 1; 2; 3; 4; 5; 6; 7;
In total 7 binding sites of Potassium where determined in the Inter-Dimeric Interface Controls Function and Stability of S- Methionine Adenosyltransferase From U. Urealiticum, PDB code: 6rkc:
Jump to Potassium binding site number: 1; 2; 3; 4; 5; 6; 7;
Potassium binding site 1 out of 7 in 6rkc
Go back to
Potassium binding site 1 out
of 7 in the Inter-Dimeric Interface Controls Function and Stability of S- Methionine Adenosyltransferase From U. Urealiticum
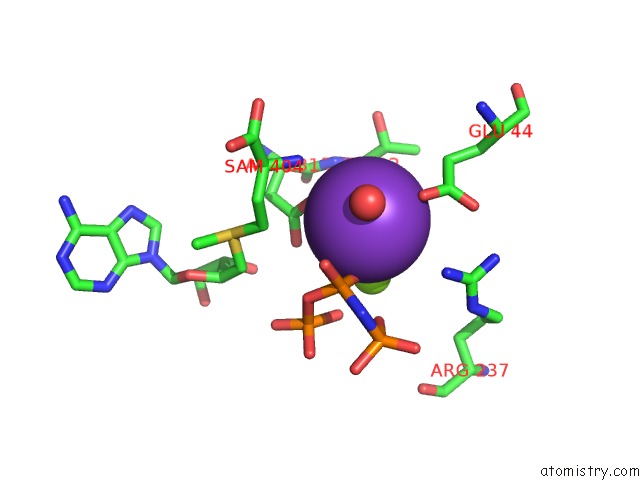
Mono view
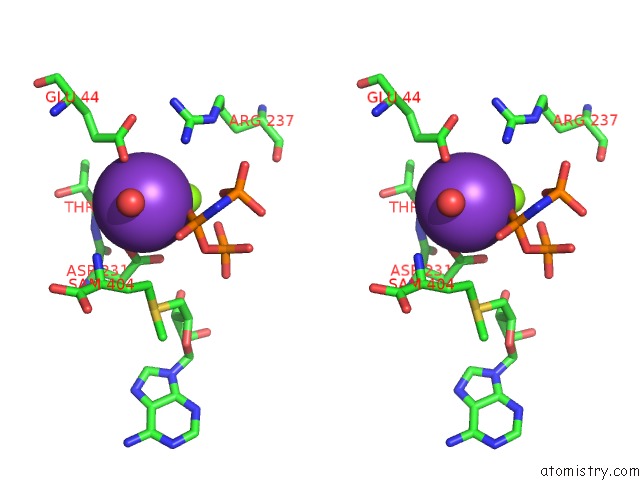
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 1 of Inter-Dimeric Interface Controls Function and Stability of S- Methionine Adenosyltransferase From U. Urealiticum within 5.0Å range:
|
Potassium binding site 2 out of 7 in 6rkc
Go back to
Potassium binding site 2 out
of 7 in the Inter-Dimeric Interface Controls Function and Stability of S- Methionine Adenosyltransferase From U. Urealiticum
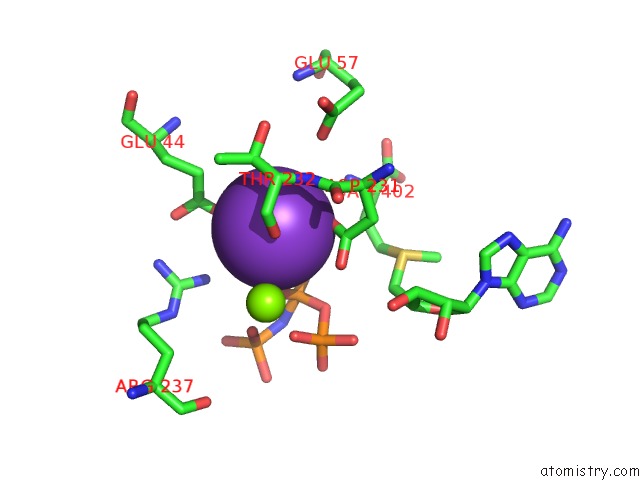
Mono view
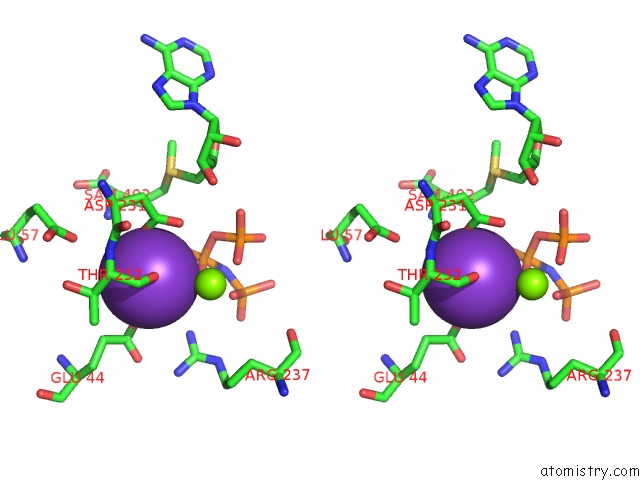
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 2 of Inter-Dimeric Interface Controls Function and Stability of S- Methionine Adenosyltransferase From U. Urealiticum within 5.0Å range:
|
Potassium binding site 3 out of 7 in 6rkc
Go back to
Potassium binding site 3 out
of 7 in the Inter-Dimeric Interface Controls Function and Stability of S- Methionine Adenosyltransferase From U. Urealiticum
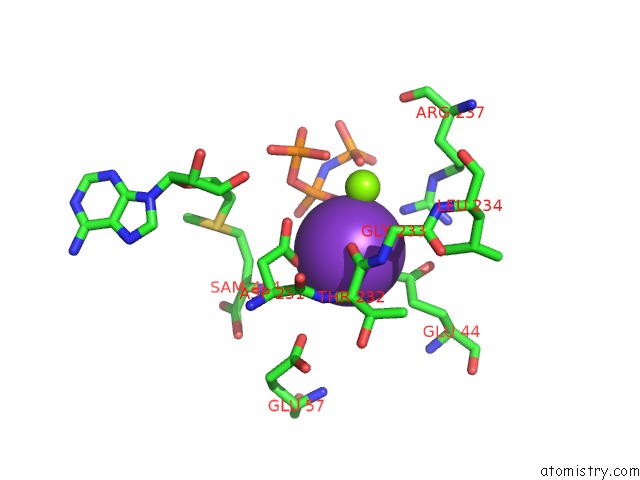
Mono view
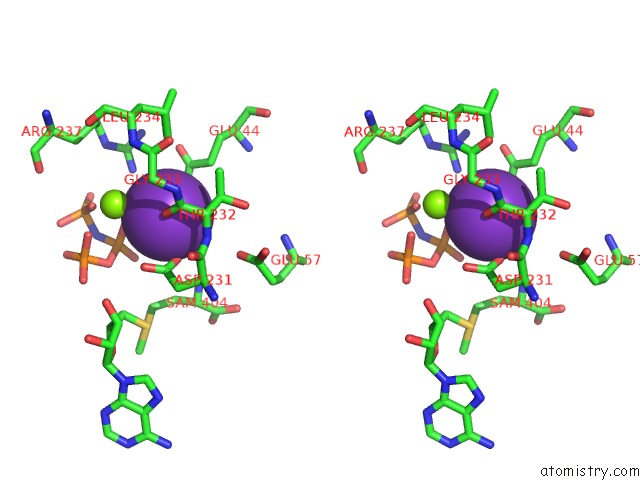
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 3 of Inter-Dimeric Interface Controls Function and Stability of S- Methionine Adenosyltransferase From U. Urealiticum within 5.0Å range:
|
Potassium binding site 4 out of 7 in 6rkc
Go back to
Potassium binding site 4 out
of 7 in the Inter-Dimeric Interface Controls Function and Stability of S- Methionine Adenosyltransferase From U. Urealiticum
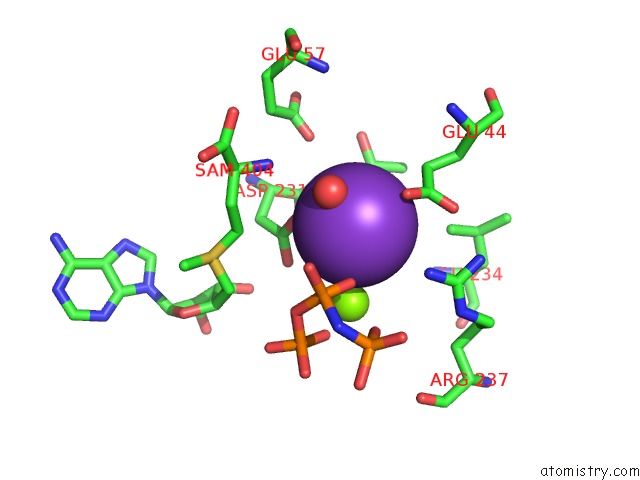
Mono view
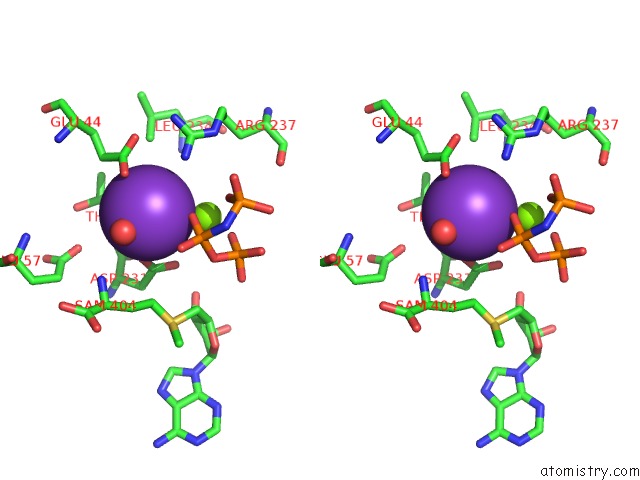
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 4 of Inter-Dimeric Interface Controls Function and Stability of S- Methionine Adenosyltransferase From U. Urealiticum within 5.0Å range:
|
Potassium binding site 5 out of 7 in 6rkc
Go back to
Potassium binding site 5 out
of 7 in the Inter-Dimeric Interface Controls Function and Stability of S- Methionine Adenosyltransferase From U. Urealiticum
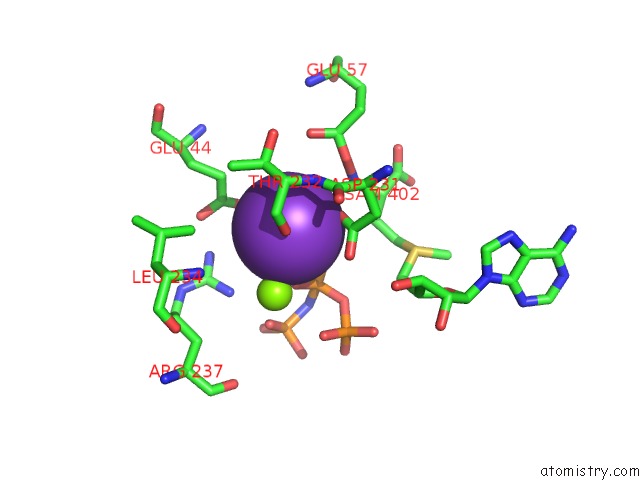
Mono view
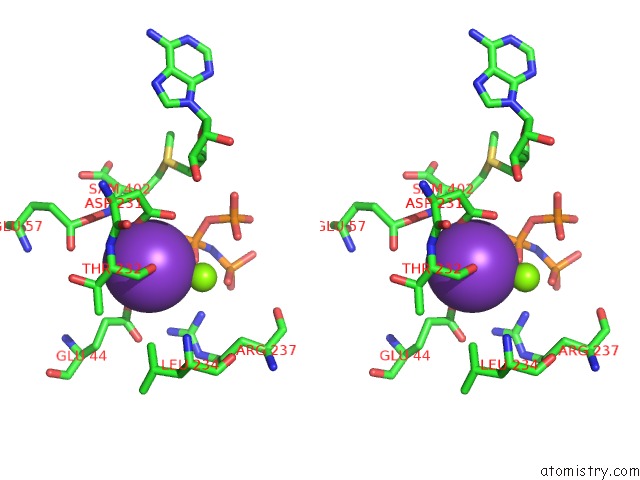
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 5 of Inter-Dimeric Interface Controls Function and Stability of S- Methionine Adenosyltransferase From U. Urealiticum within 5.0Å range:
|
Potassium binding site 6 out of 7 in 6rkc
Go back to
Potassium binding site 6 out
of 7 in the Inter-Dimeric Interface Controls Function and Stability of S- Methionine Adenosyltransferase From U. Urealiticum
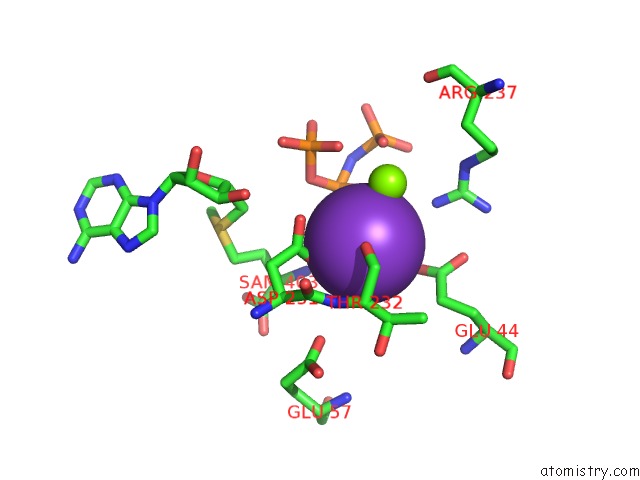
Mono view
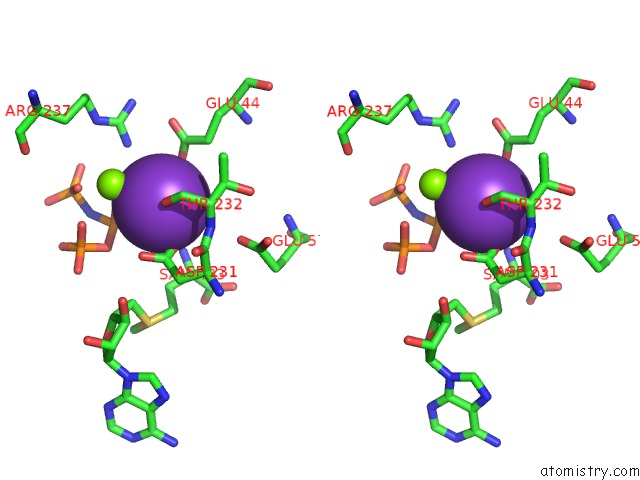
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 6 of Inter-Dimeric Interface Controls Function and Stability of S- Methionine Adenosyltransferase From U. Urealiticum within 5.0Å range:
|
Potassium binding site 7 out of 7 in 6rkc
Go back to
Potassium binding site 7 out
of 7 in the Inter-Dimeric Interface Controls Function and Stability of S- Methionine Adenosyltransferase From U. Urealiticum
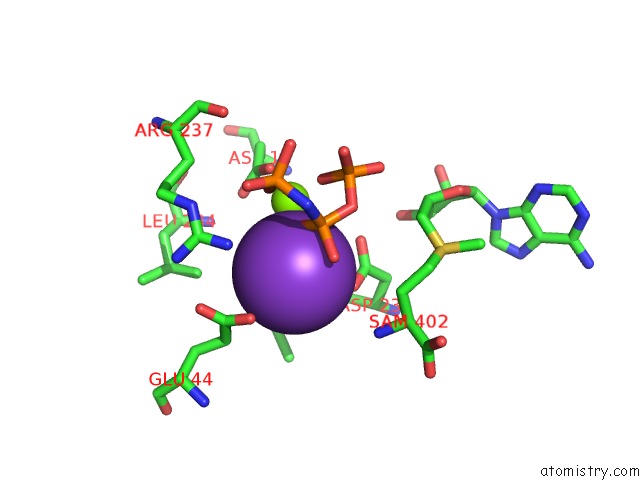
Mono view
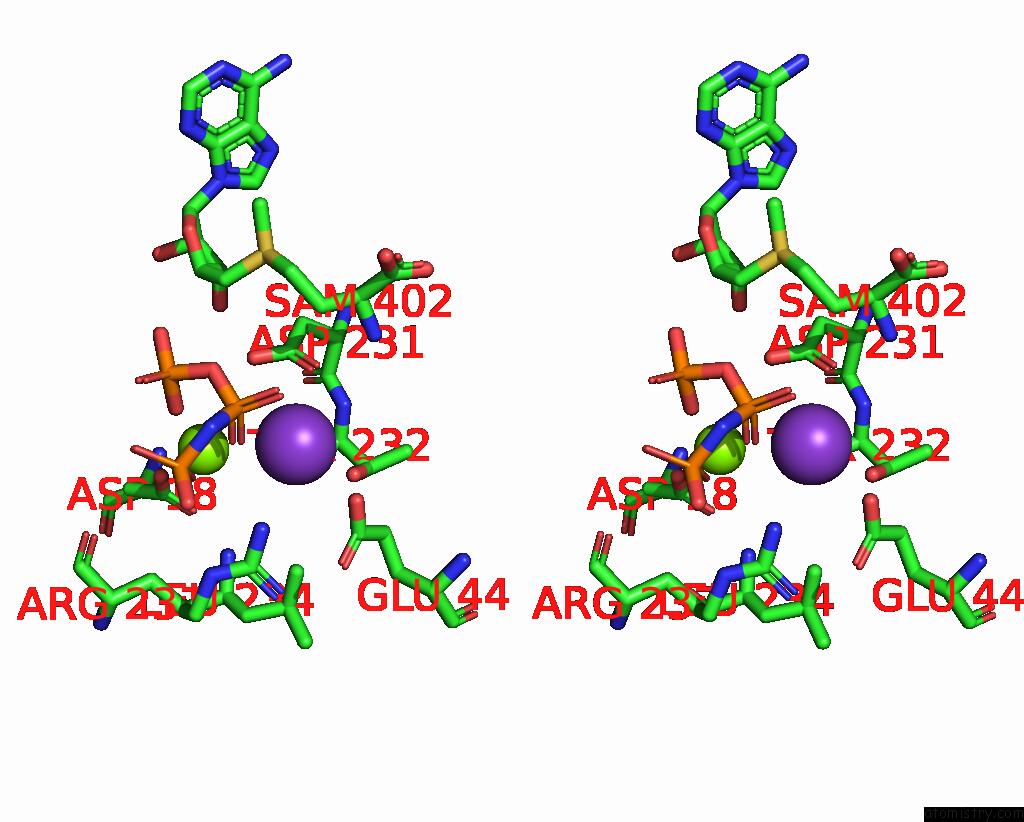
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 7 of Inter-Dimeric Interface Controls Function and Stability of S- Methionine Adenosyltransferase From U. Urealiticum within 5.0Å range:
|
Reference:
D.Kleiner,
F.Shmulevich,
R.Zarivach,
A.Shahar,
M.Sharon,
G.Ben-Nissan,
S.Bershtein.
The Inter-Dimeric Interface Controls Function and Stability of Ureaplasma Urealiticum Methionine S-Adenosyltransferase. J.Mol.Biol. 2019.
ISSN: ESSN 1089-8638
PubMed: 31520601
DOI: 10.1016/J.JMB.2019.09.003
Page generated: Sat Aug 9 12:02:33 2025
ISSN: ESSN 1089-8638
PubMed: 31520601
DOI: 10.1016/J.JMB.2019.09.003
Last articles
Mg in 1MZYMg in 1MZP
Mg in 1MXG
Mg in 1MXC
Mg in 1MXB
Mg in 1MXA
Mg in 1MWM
Mg in 1MUW
Mg in 1MV5
Mg in 1MUM