Potassium »
PDB 2hzv-2o84 »
2l8m »
Potassium in PDB 2l8m: Reduced and Co-Bound Cytochrome P450CAM (CYP101A1)
Enzymatic activity of Reduced and Co-Bound Cytochrome P450CAM (CYP101A1)
All present enzymatic activity of Reduced and Co-Bound Cytochrome P450CAM (CYP101A1):
1.14.15.1;
1.14.15.1;
Other elements in 2l8m:
The structure of Reduced and Co-Bound Cytochrome P450CAM (CYP101A1) also contains other interesting chemical elements:
| Iron | (Fe) | 1 atom |
| Chlorine | (Cl) | 3 atoms |
Potassium Binding Sites:
The binding sites of Potassium atom in the Reduced and Co-Bound Cytochrome P450CAM (CYP101A1)
(pdb code 2l8m). This binding sites where shown within
5.0 Angstroms radius around Potassium atom.
In total 4 binding sites of Potassium where determined in the Reduced and Co-Bound Cytochrome P450CAM (CYP101A1), PDB code: 2l8m:
Jump to Potassium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Potassium where determined in the Reduced and Co-Bound Cytochrome P450CAM (CYP101A1), PDB code: 2l8m:
Jump to Potassium binding site number: 1; 2; 3; 4;
Potassium binding site 1 out of 4 in 2l8m
Go back to
Potassium binding site 1 out
of 4 in the Reduced and Co-Bound Cytochrome P450CAM (CYP101A1)
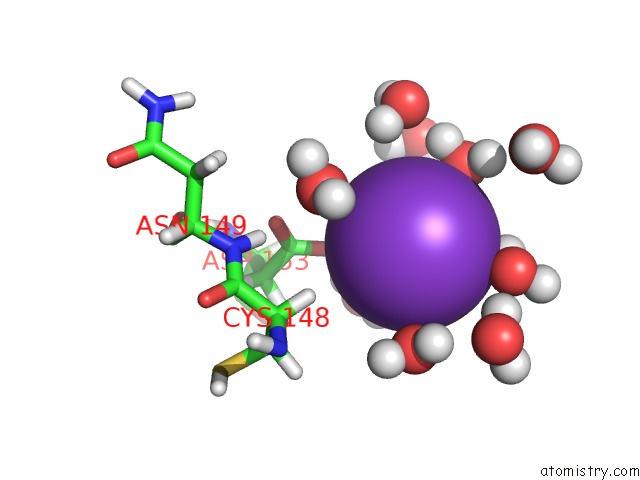
Mono view
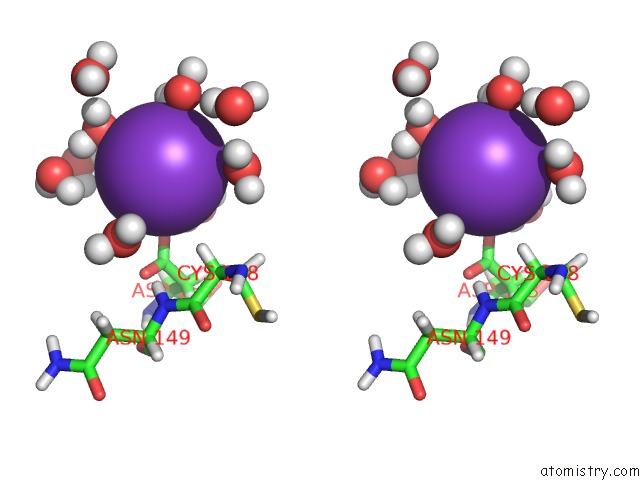
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 1 of Reduced and Co-Bound Cytochrome P450CAM (CYP101A1) within 5.0Å range:
|
Potassium binding site 2 out of 4 in 2l8m
Go back to
Potassium binding site 2 out
of 4 in the Reduced and Co-Bound Cytochrome P450CAM (CYP101A1)
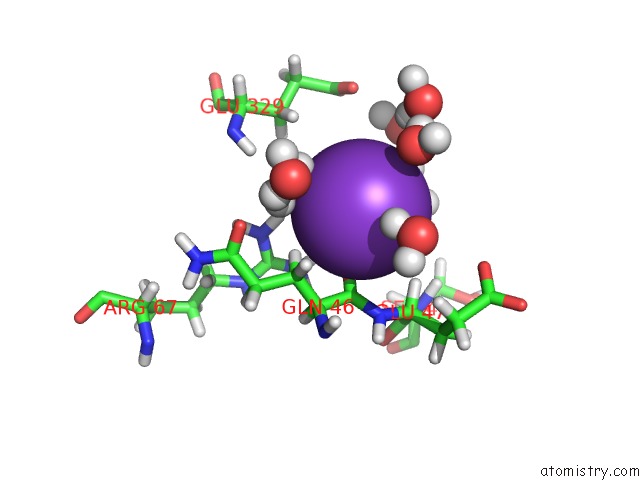
Mono view
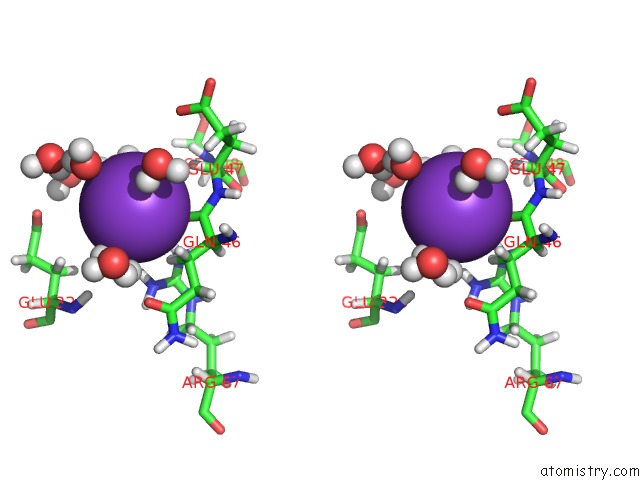
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 2 of Reduced and Co-Bound Cytochrome P450CAM (CYP101A1) within 5.0Å range:
|
Potassium binding site 3 out of 4 in 2l8m
Go back to
Potassium binding site 3 out
of 4 in the Reduced and Co-Bound Cytochrome P450CAM (CYP101A1)
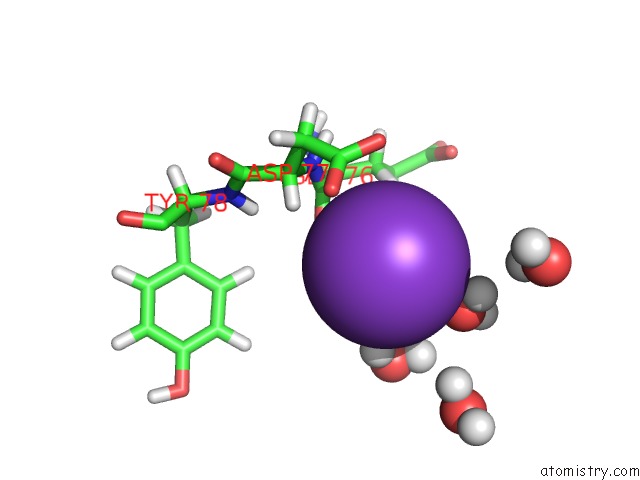
Mono view
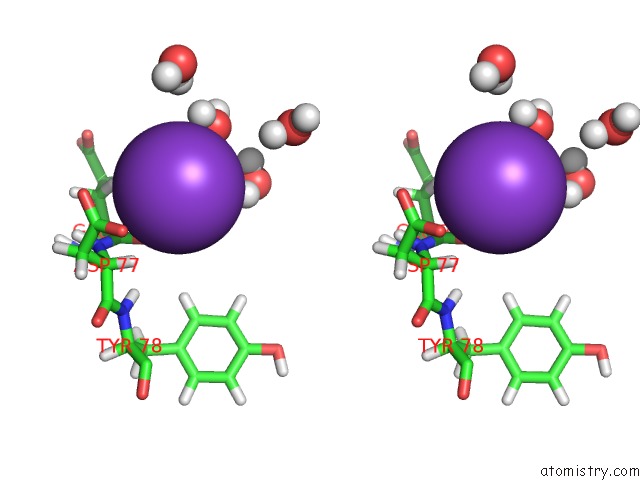
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 3 of Reduced and Co-Bound Cytochrome P450CAM (CYP101A1) within 5.0Å range:
|
Potassium binding site 4 out of 4 in 2l8m
Go back to
Potassium binding site 4 out
of 4 in the Reduced and Co-Bound Cytochrome P450CAM (CYP101A1)
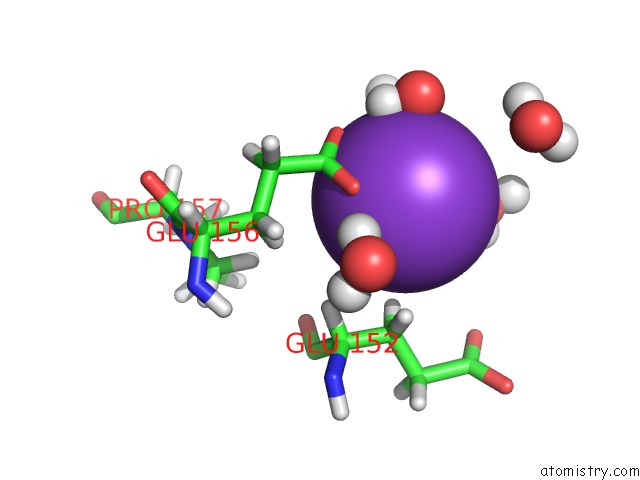
Mono view
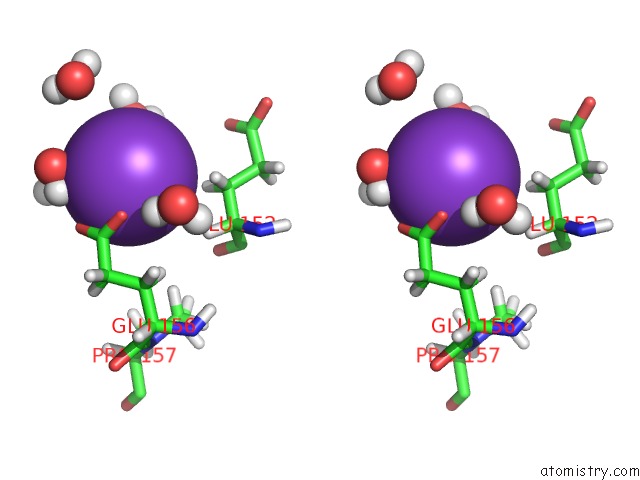
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 4 of Reduced and Co-Bound Cytochrome P450CAM (CYP101A1) within 5.0Å range:
|
Reference:
E.K.Asciutto,
M.Dang,
S.S.Pochapsky,
J.D.Madura,
T.C.Pochapsky.
Experimentally Restrained Molecular Dynamics Simulations For Characterizing the Open States of Cytochrome P450(Cam). Biochemistry V. 50 1664 2011.
ISSN: ISSN 0006-2960
PubMed: 21265500
DOI: 10.1021/BI101820D
Page generated: Sat Aug 9 03:43:11 2025
ISSN: ISSN 0006-2960
PubMed: 21265500
DOI: 10.1021/BI101820D
Last articles
K in 5EFKK in 5EHH
K in 5EFH
K in 5EFG
K in 5EFJ
K in 5EF8
K in 5EFB
K in 5EF7
K in 5EEN
K in 5EEI