Potassium »
PDB 2adp-2c13 »
2aop »
Potassium in PDB 2aop: Sulfite Reductase: Reduced with Crii Edta, Siroheme Feii, [4FE-4S] +1, Phosphate Bound
Enzymatic activity of Sulfite Reductase: Reduced with Crii Edta, Siroheme Feii, [4FE-4S] +1, Phosphate Bound
All present enzymatic activity of Sulfite Reductase: Reduced with Crii Edta, Siroheme Feii, [4FE-4S] +1, Phosphate Bound:
1.8.1.2;
1.8.1.2;
Protein crystallography data
The structure of Sulfite Reductase: Reduced with Crii Edta, Siroheme Feii, [4FE-4S] +1, Phosphate Bound, PDB code: 2aop
was solved by
B.R.Crane,
E.D.Getzoff,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 10.00 / 1.75 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 69.800, 77.400, 87.800, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 17.5 / n/a |
Other elements in 2aop:
The structure of Sulfite Reductase: Reduced with Crii Edta, Siroheme Feii, [4FE-4S] +1, Phosphate Bound also contains other interesting chemical elements:
| Iron | (Fe) | 5 atoms |
Potassium Binding Sites:
The binding sites of Potassium atom in the Sulfite Reductase: Reduced with Crii Edta, Siroheme Feii, [4FE-4S] +1, Phosphate Bound
(pdb code 2aop). This binding sites where shown within
5.0 Angstroms radius around Potassium atom.
In total only one binding site of Potassium was determined in the Sulfite Reductase: Reduced with Crii Edta, Siroheme Feii, [4FE-4S] +1, Phosphate Bound, PDB code: 2aop:
In total only one binding site of Potassium was determined in the Sulfite Reductase: Reduced with Crii Edta, Siroheme Feii, [4FE-4S] +1, Phosphate Bound, PDB code: 2aop:
Potassium binding site 1 out of 1 in 2aop
Go back to
Potassium binding site 1 out
of 1 in the Sulfite Reductase: Reduced with Crii Edta, Siroheme Feii, [4FE-4S] +1, Phosphate Bound
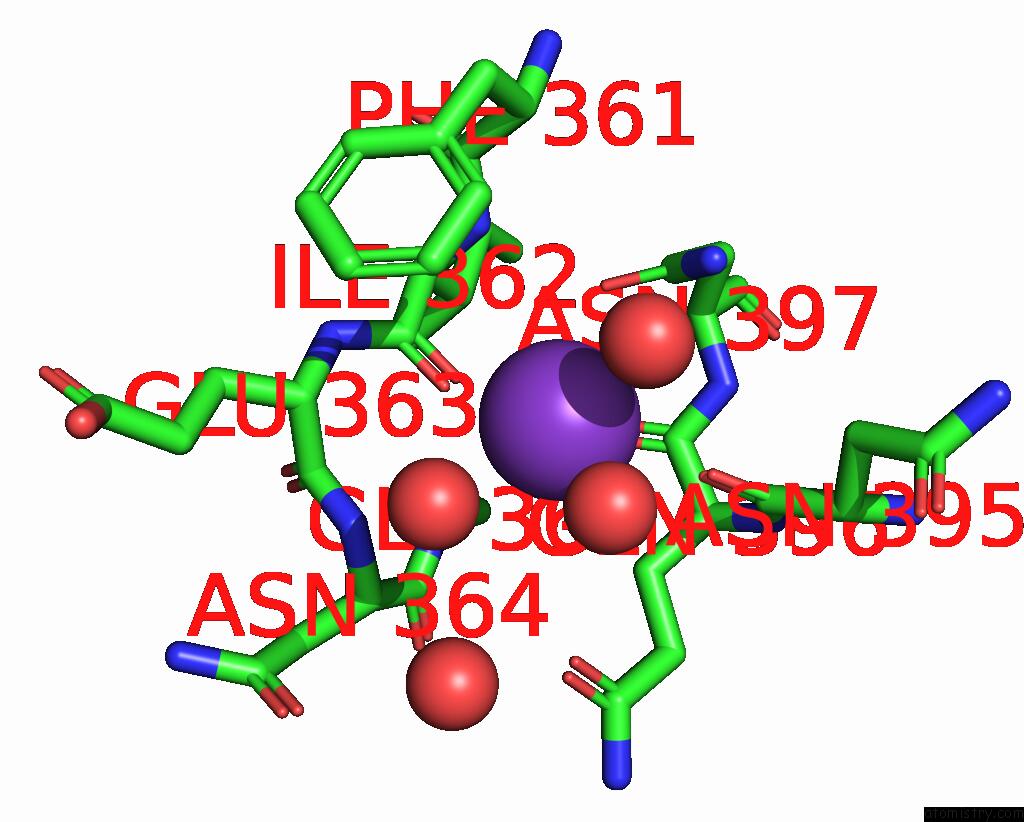
Mono view
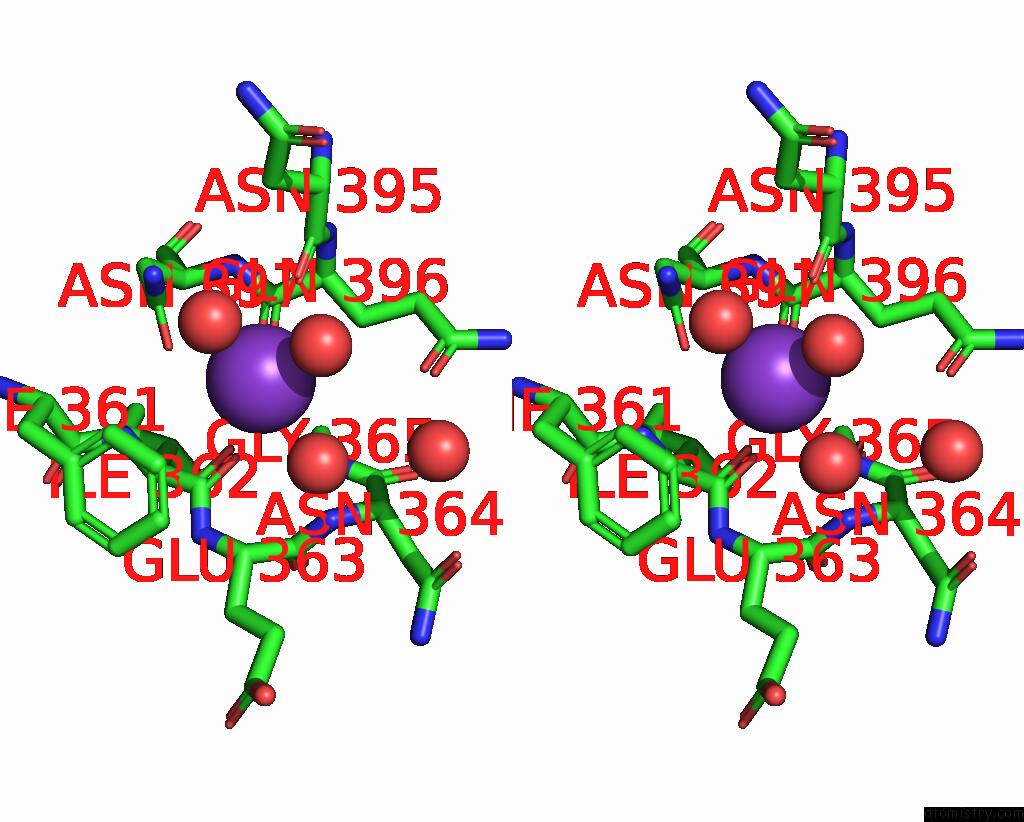
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 1 of Sulfite Reductase: Reduced with Crii Edta, Siroheme Feii, [4FE-4S] +1, Phosphate Bound within 5.0Å range:
|
Reference:
B.R.Crane,
L.M.Siegel,
E.D.Getzoff.
Structures of the Siroheme- and FE4S4-Containing Active Center of Sulfite Reductase in Different States of Oxidation: Heme Activation Via Reduction-Gated Exogenous Ligand Exchange. Biochemistry V. 36 12101 1997.
ISSN: ISSN 0006-2960
PubMed: 9315848
DOI: 10.1021/BI971065Q
Page generated: Sat Aug 9 03:07:25 2025
ISSN: ISSN 0006-2960
PubMed: 9315848
DOI: 10.1021/BI971065Q
Last articles
Mg in 3G5AMg in 3G5S
Mg in 3G58
Mg in 3G4T
Mg in 3G4L
Mg in 3G4K
Mg in 3G4I
Mg in 3G4G
Mg in 3G4F
Mg in 3G45