Potassium »
PDB 1u1g-1w29 »
1vqn »
Potassium in PDB 1vqn: The Structure of Cc-Hpmn and Cca-Phe-Cap-Bio Bound to the Large Ribosomal Subunit of Haloarcula Marismortui
Protein crystallography data
The structure of The Structure of Cc-Hpmn and Cca-Phe-Cap-Bio Bound to the Large Ribosomal Subunit of Haloarcula Marismortui, PDB code: 1vqn
was solved by
T.M.Schmeing,
T.A.Steitz,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 50.00 / 2.40 |
| Space group | C 2 2 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 211.721, 298.782, 575.272, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 21.2 / 24.8 |
Other elements in 1vqn:
The structure of The Structure of Cc-Hpmn and Cca-Phe-Cap-Bio Bound to the Large Ribosomal Subunit of Haloarcula Marismortui also contains other interesting chemical elements:
| Strontium | (Sr) | 114 atoms |
| Magnesium | (Mg) | 94 atoms |
| Cadmium | (Cd) | 5 atoms |
| Chlorine | (Cl) | 22 atoms |
| Sodium | (Na) | 75 atoms |
Potassium Binding Sites:
The binding sites of Potassium atom in the The Structure of Cc-Hpmn and Cca-Phe-Cap-Bio Bound to the Large Ribosomal Subunit of Haloarcula Marismortui
(pdb code 1vqn). This binding sites where shown within
5.0 Angstroms radius around Potassium atom.
In total 2 binding sites of Potassium where determined in the The Structure of Cc-Hpmn and Cca-Phe-Cap-Bio Bound to the Large Ribosomal Subunit of Haloarcula Marismortui, PDB code: 1vqn:
Jump to Potassium binding site number: 1; 2;
In total 2 binding sites of Potassium where determined in the The Structure of Cc-Hpmn and Cca-Phe-Cap-Bio Bound to the Large Ribosomal Subunit of Haloarcula Marismortui, PDB code: 1vqn:
Jump to Potassium binding site number: 1; 2;
Potassium binding site 1 out of 2 in 1vqn
Go back to
Potassium binding site 1 out
of 2 in the The Structure of Cc-Hpmn and Cca-Phe-Cap-Bio Bound to the Large Ribosomal Subunit of Haloarcula Marismortui
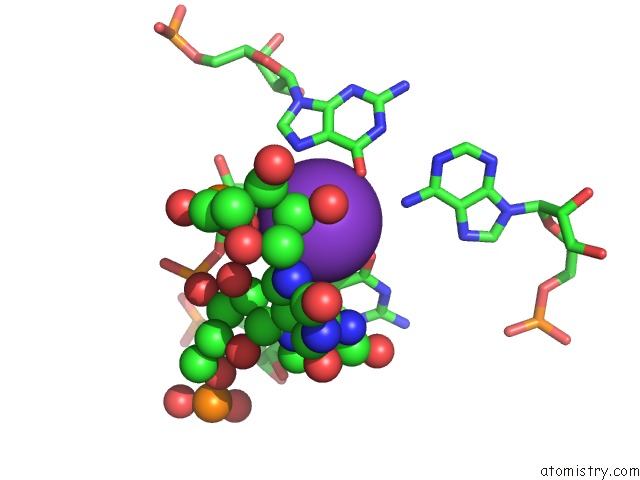
Mono view
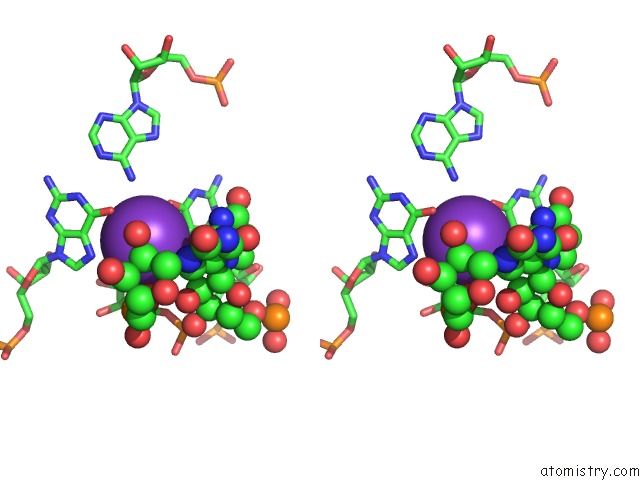
Stereo pair view

Mono view

Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 1 of The Structure of Cc-Hpmn and Cca-Phe-Cap-Bio Bound to the Large Ribosomal Subunit of Haloarcula Marismortui within 5.0Å range:
|
Potassium binding site 2 out of 2 in 1vqn
Go back to
Potassium binding site 2 out
of 2 in the The Structure of Cc-Hpmn and Cca-Phe-Cap-Bio Bound to the Large Ribosomal Subunit of Haloarcula Marismortui
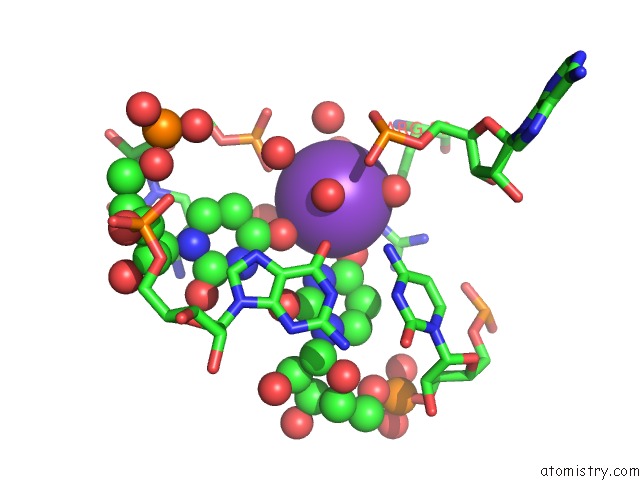
Mono view
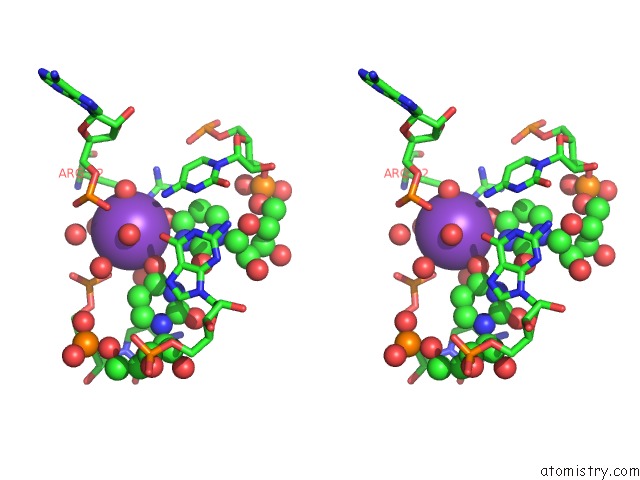
Stereo pair view

Mono view
Stereo pair view
A full contact list of Potassium with other atoms in the K binding
site number 2 of The Structure of Cc-Hpmn and Cca-Phe-Cap-Bio Bound to the Large Ribosomal Subunit of Haloarcula Marismortui within 5.0Å range:
|
Reference:
T.M.Schmeing,
K.S.Huang,
S.A.Strobel,
T.A.Steitz.
An Induced-Fit Mechanism to Promote Peptide Bond Formation and Exclude Hydrolysis of Peptidyl-Trna. Nature V. 438 520 2005.
ISSN: ISSN 0028-0836
PubMed: 16306996
DOI: 10.1038/NATURE04152
Page generated: Mon Aug 12 05:39:26 2024
ISSN: ISSN 0028-0836
PubMed: 16306996
DOI: 10.1038/NATURE04152
Last articles
As in 4JSMAs in 4K4G
As in 4JSL
As in 4JSK
As in 4JLH
As in 4JD0
As in 4J9T
As in 4JQW
As in 4J8M
As in 4IKB